Research - (2023) Volume 11, Issue 3
The mean duration of sleep in mice increased with an increased dose of the crude ethanolic extract of Hippocrateae welwitschii. The mice dosed with the extract all dropped in an open field and couldn’t maintain balance at a 45o inclined plane. These imply that the extract is sedative and affected their motor coordination. The ethanolic root extract of Hippocrateae welwitschii reduced the onset of seizure in extract-treated mice dosed with flumazenil by 0-40% (p<0.05). In addition, flumazenil completely blocked the protection provided by diazepam against seizure while the protection against seizure in Hippocratea welwitschii extract-treated mice was 20% in the presence of naloxone (opioid receptor). The fact that flumazenil reduced seizure onset and attenuated the protection against seizure provided by Hippocratea welwitschii suggest that it may contain substance(s) that interacted with the benzodiazepine site in the GABAA-benzodiazepine receptor complex. The ability of the extract to exhibit activity against these two types of seizures suggests that it may act through different mechanisms to elicit its anticonvulsant effects, such as voltage-gated sodium, calcium, and potassium or GABA-ergic pathway. The biphasic activity observed in Pentylenetetrazole (PTZ) induced studies may probably be due to the possible interaction between constituents of the crude extract.
Sedative • Intra-peritoneal • Anticonvulsant • Biphasic • Benzodiazepine
The name “epilepsy” is derived from the Greek word “epilambanein”, which means, “to attack” or “to seize upon” [1]. Epilepsy is a seizure disorder that may be partial or total [1]. It is one of the oldest neurological disorders known to man. A seizure can be described as, a convulsive episode, which may be generalized or partial. Seizures are primarily classified relative to where they originate from and the site they affect within the brain. It may start as atypical, excessive hyper-synchronous discharge from a group of neurons in the brain before it mops up surrounding neurons to one side of the brain (partial seizures), or may spread to nerve cells throughout the brain (generalized seizures) [1]. Epilepsy is the next most frequent neurodegenerative disease after stroke and it is one of the most common, chronic neurological disorders, characterized by recurrent and spontaneous brain seizures [1].
There are over 40 different types of epilepsy, with each presenting its own unique combination of seizure type, typical age of onset, EEG (electroencephalogram) findings, treatment, and prognosis [2]. The incidence of epilepsy is worldwide but its prevalence ranges from 0.5%-1.0 % of the world population with the industrialized countries having less and Africa more [2]. In new patients diagnosed with epilepsy, almost 30% of they have seizures originating from the Temporal Lobe of the brain (TLE) [1]. Individuals affected with TLE characteristically have similar clinical afflictions, including an Initial Precipitating Injury (IPI) such as Status Epilepticus (SE), head trauma, encephalitis, or childhood febrile seizures [1]. Patients seem to exhibit a latent period of several years between this injury and the emergence of the chronic TLE characterized by Spontaneous Recurrent Motor Seizures (SRMS) originating from temporal lobe foci, and learning and memory impairments. TLE is also frequently associated with hippocampal sclerosis, typified by advanced neuro-degeneration in the Dentate Hilus (DH), and the CA1 and CA3c subregions [3]. Human studies suggest that hippocampal sclerosis likely initiates or contributes to the generation of most TLEs [1].
About 25% of people diagnosed with epilepsy have seizures that cannot be controlled by antiepileptic drugs [4,5]. However, there are various types of therapeutic approaches that aim to prevent epileptogenesis after the SE or an IPI. Extensive efforts have been made to achieve neuroprotection through effective seizure suppression with Antiepileptic Drugs (AEDs). The anticonvulsive mechanisms of conventional and newly introduced drugs vary considerably. It has been reported that most of AEDs work through biphasic mechanisms and most of them have undesirable side effects [4]. The most common actions were shown to affect ion channels, GABA-ergic and glutamatergic metabolism, receptors, or secondary messengers [5]. Various reports suggest that conventional as well as recently introduced anticonvulsants have some neuroprotective activity in models of ischemia [6,7].
During partial seizures, nerve discharge in a localized area of the brain is affected, while in generalized seizures, nerve discharge through the whole brain is affected. Partial seizures may be further described in the context of if the state of consciousness was not affected (simple partial) or if consciousness was affected (complex partial). Partial seizures that spread within the brain occur through a process known as secondary generalization. Temporal Lobe Epilepsy (TLE) encompasses partial seizures and is among the most frequent types of epilepsy [1]. Generalized seizures are further described according to the extent the body is affected, however, all involve loss of consciousness. These include but are not limited to the absence (petit mal), myoclonic, clonic, tonic, tonic-clonic (grand mal), and atonic seizures
Episodes of seizures may cause extensive brain damage which is a dynamic process that encompasses multiple factors leading to neuronal cell death. These may also include genetic factors, glutamate-mediated toxicity leading to interruptions in the intracellular electrolyte metabolism, oxidative stress, mitochondrial dysfunction, growth factor depletion, and increased concentration of cytokines [2]. Intense seizure activity also initiates a massive influx of calcium via voltage-gated and N-Methyl-D-Aspartate (NMDA)-dependent ion channels [3]. Elevated calcium levels within the cell result in the activation of biochemical cascades which trigger acute neuronal cell death after the seizure episodes and may induce the generation of free radicals [2,3].
The consequences of initial seizures in humans are difficult to predict due to multiple series of factors such as differences in the etiology and age at the onset of seizures, the types, frequency, and duration of seizures, and genetic predisposition [7]. However, it has been well established that epilepsy or seizures are linked with neurodegeneration in several areas of the brain [8]. It is clear that both necrotic and apoptotic cell death contributes to neuronal damage in partial seizures and epileptogenic insults such as SE, head injury, or stroke [3,9]. It is supposed that multiple mechanisms and neurochemical modulators play roles in the initiation of epileptogenesis after an IPI. Epileptogenesis is the transformation of the normal neuronal network into a long-lasting chronically hyper excitable state [9]. Understanding the action pathway of any potential antiepilepsy drug formula is very important in order to ensure the appropriate handling of patients.
This study therefore, seeks to investigate the sedative, anticonvulsant potentials and the mechanism of action pathway of the ethanolic root extract of Hippocrateae welwitschii which has been reported to reduce the onset of seizure in extract-treated mice dosed with flumazenil from 0%-40% (p<0.05) [2].
Determination of the sedative attribute of the ethanolic root extract of H.welwitschii and motor coordination of sedated mice
Diazepam was used to sedate the mice. It has been known to abort seizures by depressing the central nervous system when they occur or prevent them from occurring in some cases. The extract (200.0 mg/kg, 400.0 mg/kg, 800.0 mg/kg) body weight were administered respectively 30 mins before diazepam, as shown seen in Figures 1-4 and Table 1 below. The behavior of the sedated mice is shown in Figure 5. The open field and inclined plane at 45° inclined plane were observed.
Showing sedated mice.
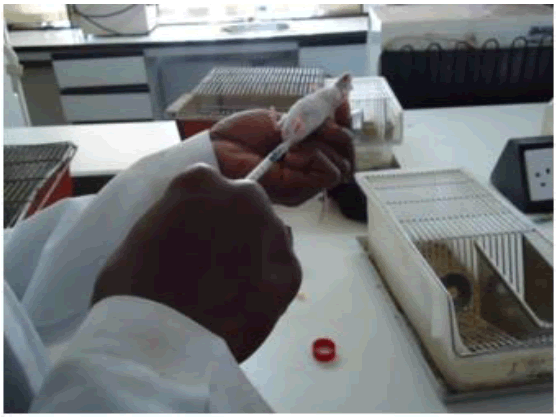
Figure 1: Dosing of mice with diazepam sub-cutaneous after 30 mins of oral dozing with Hw ethanolic crude extract (200 mg/kg).

Figure 2: Sedated with only diazepam (control).

Figure 3: Extract sedated mice (400 mg/kg).

Figure 4: Sedated and sleeping mice (800 mg/kg).
Table 1. Sedative analysis of the crude ethanolic root extract of Hippocratea welwithscii on albino mice.
| Dose | Code | T0 | T1 | T2 | TW | DS | Mean T2 | Mean DS |
|---|---|---|---|---|---|---|---|---|
| 200 mg/kg | TL | 9.42 | 10.3 | 10.4 | 12.5 | 137 | 14.2 ± 0.18 | 86 ± 14.10 |
| TLRS | 10.3 | 10.4 | 11.6 | 74 | ||||
| TLLS | 10.3 | 10.4 | 12.1 | 90 | ||||
| LLSTRP | 10.3 | 10.4 | 12.1 | 90 | ||||
| LL | 10.3 | 10.5 | 12.2 | 89 | ||||
| 400 mg/kg | HDBK | 9.44 | 10.3 | 10.5 | 1.47 | 182 | 8.8 ± 1.89 | 162.2 ± 46 |
| HDLA | 10.3 | 10.4 | 12.6 | 136 | ||||
| TLRA | 10.3 | 10.4 | 1.31 | 174 | ||||
| HDRA | 10.4 | 10.5 | 1.07 | 140 | ||||
| BK | 10.4 | 10.4 | 1.36 | 179 | ||||
| 800 mg/kg | RA | 9.49 | 10.4 | 10.5 | 1.04 | 236 | 6.6 ±1.28 | 182.4 ± 52.74 |
| LA | 10.4 | 10.4 | 12.2 | 293 | ||||
| HDRS | 10.4 | 10.4 | 11.5 | 262 | ||||
| RS | 10.4 | 10.5 | 4.18 | 329 | ||||
| HD | 10.4 | 10.5 | 2.38 | 232 | ||||
| Diazepam 3 mg/kg | HDLS | 10.6 | 10.6 | 11.2 | 27 | 6.4 ± 1.00 | 76.0 ± 13.21 | |
| RL | 10.5 | 12.4 | 112 | |||||
| RALA | 10.5 | 12.3 | 101 | |||||
| BKRA | 10.6 | 12.1 | 69 | |||||
| BKLL | 10.6 | 12.1 | 71 | |||||
| T0=Time of dosing with crude extract; T1=Time of injecting with diazepam, T2=Time of onset of sleep, TW=Time the mice woke, DS=Duration of sleep in minutes, Mean T2=Mean onset of sleep and Mean DS=Mean duration of sleep | ||||||||
Motor coordination of sedated mice.

Figure 5: The mice became immobile, sedated, and dropped into an open field.
Anticonvulsant effect of H.welwitschiiroot ethanolic extract on PTZ induced convulsions in mice
The method of Swinyard et al (1989) was employed to induce convulsion in mice using PTZ [10]. Sixty mice were divided into six groups each containing ten mice (Figures 6-10). The first group received normal saline with 10 ml/kg body weight, the second, third, fourth, and fifth groups received 200, 400 mg/kg, 600 mg/kg, and 800 mg/kg body weight ad labitium of ethanol extract of Hippocrateae welwitschii while the sixth group was injected with diazepam 3 mg/kg body weight IP. Thirty minutes after treatment, mice in all the groups received PTZ 85 mg/kg Subcutaneous (Sc) and were observed over a period of 30 min (Figures 6-10).

Figure 6: Sorted mice in cages
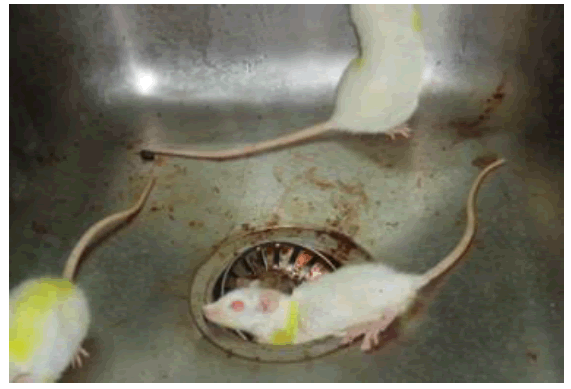
Figure 7: Glassy eyes, stretching, and hyper-ventilation preceding convulsion.

Figure 8: Hyperventilation, glazy eyes, hyperactivity.

Figure 9: Seizure in progress (BKRL) while LA was protected.

Figure 10:Strobe reaction (NM) and hind limb tonic extension (BKSTR) in response to tonic seizures of induced mice.
Anti-epileptic properties of H.welwitschii ethanolic root extract: Figures 6- 10. After treatment, mice in all the groups received PTZ 85 mg/kg Subcutaneous (Sc) and were observed over a period of 30 min.
• NM- Mouse with no mark.
• BKSTR- Mouse with a back strip.
• BKRL- Back right leg.
• LA- Left arm.
Mechanistic study of action pathway of ethanolic root extract of Hippocratea welwitschii
In order to elucidate the mechanism involved in Hippocratea welwitschii induced partial protection of mice from PTZ-induced seizure,
• Flumazenil
• Naloxone was used.
Mechanistic study of anticonvulsant effect of Hippocratea welwitschii ethanolic crude extract against PTZ induced seizure in mice via GABA-ergicbenzodiazepine mechanism: Flumazenil+H.W.+PTZ: Flumazenil (10 mg/kg),an antagonist of benzodiazepine (BZD) site in the GABAA - BZD receptor complex was used prior to injection of Hippocratea welwitschii extract (200 mg/kg) or diazepam (3 mg/kg).
Mechanistic study of naloxone (opiod receptor) on the anticonvulsant attributes of ethanolic root extract of Hippocratea welwitschii in Pentylenetetrazole (PTZ)-induced seizure in mice. Naloxone+H.W+PTZ:
Naloxone (3 mg/kg), an opioid receptor antagonist was administered 15 min before the Hippocratea welwitschii ethanolic extract. PTZ (85 mg/kg) was injected 30 minutes after the administration of Hippocratea welwitschii; values are the mean ± SEM of five mice.
In the anticonvulsant effect of Hippocratea welwitschii root extract on PTZinduced convulsions in mice, the absence of an episode of clonic spasm of at least 5 seconds duration would indicate the extract’s ability to abolish the effect of PTZ on seizure threshold. After treatment, mice in all the groups received PTZ 85 mg/kg Subcutaneous (Sc) and were observed over a period of 30 min (Table 2).
Table 2. Anticonvulsant effect of H.W on PTZ-induced seizure in mice.
| Dose(mg/kg) | ID | WT in (g) | T1 | Onset of clonus (sec) | Mean onset of clonus (secs) | HLTE | Mean HLTE sec | Survival (D/A) | % protection |
|---|---|---|---|---|---|---|---|---|---|
| 85 mg/kg of PTZ + normal saline | BKLA | 18 | 10.13am | 47 | 48 | 195 | 207 | D | 0% |
| HDLL | 20 | 40 | 177 | D | |||||
| HDTL | 17 | 61 | 173 | D | |||||
| BKRL | 20 | 37 | 194 | D | |||||
| HDRS | 21 | 55 | 328 | D | |||||
| NM | 18 | 37 | 195 | D | |||||
| BK | 22 | 61 | 117 | D | |||||
| RS | 20 | 40 | 194 | D | |||||
| LA | 22 | 47 | 173 | D | |||||
| LL | 21 | 55 | 328 | D | |||||
| 200 mg/kg H.W+85 mg/kg PTZ | H.W. admin | Onset of clonus | 50% | ||||||
| HDBK | 27 | 1.37 | 87 | 80.92 | - | A | |||
| LSRL | 24 | 1.38 | 61 | 377 | D | ||||
| TLRA | 22 | 1.39 | 68 | 421 | D | ||||
| BK | 21 | 1.4 | 58 | 327 | D | ||||
| LSLL | 17 | 1.41 | 47 | 69 | 290.6 | A | |||
| HDLL | 18 | 1.42 | 180 | 620 | D | ||||
| BKRA | 23 | 1.43 | 186 | 904 | D | ||||
| LA | 29 | 1.44 | - | - | A | ||||
| LS | 24 | 1.45 | 188 | 188 | A | ||||
| LL | 24 | 1.46 | 114 | - | A | ||||
| 400 mg/kg | BKLS | 28 | 1.5 | 104 | 58.4 | 110 | D | 10% | |
| RSTL | 22 | 1.51 | 20 | 68 | A | ||||
| TLLA | 24 | 1.52 | 48 | 236 | D | ||||
| RSLL | 20 | 1.53 | 65 | 321 | D | ||||
| TLLS | 18 | 1.54 | 40 | 286 | 251.7 | D | |||
| RA | 16 | 1.55 | 90 | 848 | D | ||||
| TLRL | 25 | 1.56 | 46 | 94 | D | ||||
| BKRS | 21 | 1.57 | 66 | 88 | D | ||||
| BKLL | 25 | 1.58 | 46 | 258 | D | ||||
| RARS | 22 | 1.59 | 59 | 208 | D | ||||
| 600 mg/kg of H.W.+85 mg/kg of PTZ | RSLL | 20 | 1.2 | 49 | 73.9 | 267 | D | 60% | |
| BKTL | 22 | 1.21 | 58 | - | A | ||||
| RSLA | 25 | 1.22 | 72 | - | A | ||||
| HDLS | 18 | 1.23 | 62 | 308 | D | ||||
| RS | 19 | 1.24 | 120 | - | A | ||||
| LSLA | 29 | 1.25 | 66 | 721 | 160.4 | D | |||
| TL | 18 | 1.26 | 120 | - | A | ||||
| BK | 17 | 1.27 | 72 | - | A | ||||
| LA | 26 | 1.27 | 62 | 308 | D | ||||
| RA | 16 | 1.28 | 58 | A | |||||
| 800 mg/kg of H.W+85 mg/kg PTZ | TLLL | 30 | 1.3 | 76 | 70.2 | - | A | 40% | |
| LSLA | 23 | 1.31 | 58 | 305 | D | ||||
| HDLA | 22 | 1.32 | 99 | 109 | A | ||||
| RL | 19 | 1.34 | 80 | 789 | D | ||||
| TL | 16 | 1.35 | 48 | 163 | D | ||||
| HD | 14 | 1.36 | 52 | 174 | 236.8 | D | |||
| LSRS | 25 | 1.37 | 60 | 274 | D | ||||
| NM | 22 | 1.38 | 64 | - | A | ||||
| HDRA | 21 | 1.39 | 101 | - | A | ||||
| LARS | 25 | 1.4 | 64 | 554 | D | ||||
| 3 mg/kg diazepam + PTZ | BKLA | 19 | 0 | - | A | 100% | |||
| HDLL | 22 | 0 | - | A | |||||
| HDTL | 22 | 0 | - | A | |||||
| BKRL | 31 | 0 | - | A | |||||
| HDRS | 19 | 0 | - | A | |||||
| LA | 26 | 0 | - | A | |||||
| LL | 24 | 0 | - | A | |||||
| TL | 18 | 0 | - | A | |||||
| RL | 19 | 0 | - | A | |||||
| RS | 19 | 0 | - | A | |||||
| H.w=Hippocratea welwitschii; HLTE=Hind Limb Tonic Extension D=Dead, A=Alive | |||||||||
The first set of 10 mice identified as Back, Left Arm (BKLA) to Left Leg (LL), etc, with body weights of between 17.0 g-22.0 g were injected with 85 mg/kg of PTZ dissolved in normal saline. They had a mean onset of clonus time of 48 seconds and the time for the tonic hind limb extension ranging from 117 s to 328 s, there was zero percent protection, which means that they all died. This set served as the control. The fact that the mice that were treated with normal saline and PTZ all died means that the normal saline could not protect the mice from PTZ-induced seizures, that is 0% protection.
In Figures 1-4 above and Table 1 below, the mean onset of sleep in mice dosed with 200 mg/kg, 400 mg/kg, and 800 mg/kg body weights were 14.2 ± 0.18 minutes, 6.6 ± 1.89 minutes and 6.6 ± 1.28 minutes respectively, the mice dozed with only diazepam was 6.4 ± 1.00 minutes. The mean duration of sleep increased with an increased dose of the extract from 86 ± 14.10 for 200 mg/kg to 182.4 ± 52.74 minutes for 800 mg/kg body weights whereas that of diazepam was 76.4 ± 13.21 minutes below them all. The mice dosed with the crude ethanolic extract all dropped in an open field (pics 5-7) and couldn’t maintain balance at a 45° inclined plane. These imply that the crude ethanolic root extract of Hippocrates welwitschii is sedative and affected motor coordination, probably by depressing the Central Nervous System (CNS) like diazepam.
The second cage housed mice whose weights were between 17.0 g/kg-28.0 g/kg body weight. They were each fed orally with 200 mg/kg of extract and then injected with 85 mg/kg body weight of PTZ. This delayed the mean onset of clonus to 98.9 seconds. This means that the extract protected the mice from seizure as can be seen in figures 6-10 and table 2 below. LA had no clonus at all while HDBK and LL after the initial jerk had no other convulsions again, so they were protected but some were not protected and they convulsed and died. In all, half of the mice in this group administered with 200 mg/kg of crude extract had 50% protection. Those administered with 400 mg/kg had 10% protection but those of 600 mg/kg had 60% protection while those administered with 800 mg/kg had 40% (p< 0.05) protection. The trend suggests that the protection may not be dosedependent. However, the mice administered with just PTZ and diazepam had 100% protection. This means that diazepam aborted seizure occurrence.
Statistical significance was tested using Student’s t-test. The difference was taken to be statistically significant at p<0.05.
The result of the mechanism of action of the crude ethanolic root extract of Hippocratea welwitschii showed that its action is biphasic. That is both via the opioid receptor in the central nervous system (20% protection) and via Benzodiazepine (BZD) site in the GABAA-BZD receptor complex (40% protection).
The extract (200 mg/kg) provided 40% protection (p<0.05) which is significant, (student-t-test). In contrast, the mean onset of convulsions in PTZ-induced convulsion was increased with 400 mg/kg of Hippocratea welwitschii extract (Table 2).
Diazepam (3 mg/kg) body weight increased the mean onset of convulsion and offered 60% protection (p<0.05). Flumazenil (10 mg/kg) reduced the onset of convulsion in diazepam-treated mice as shown in table 2 and reduced the protection against seizure from 40%-0%
Similarly, the same dose of flumazenil reduced the onset of seizure in Hippocratea welwitschii extract-treated mice from 0%-40% (p<0.05). In addition, flumazenil completely blocked the protection provided by diazepam against seizure (Table 3). However, the protection against seizure in Hippocratea welwitschii extract-treated mice was 20% in the presence of naloxone (opiod receptor). The biphasic activity observed in Pentylenetetrazole (PTZ)-induced mice may probably be due to the possible interaction between constituents of the crude extract. This is supported by Wickenden et al, 2002, who reported that the majority of currently available antiepileptic drugs fall into one of two pharmacological classes; those that modulate neuronal voltage-gated sodium channels (e.g. carbamazepine, phenytoin, lamotrigine, and topiramate) and those that modulate inhibitory GABAergic neurotransmission (e.g. benzodiazepine, vigabatrin and tiagabine) while small number of AEDs such as ethosuximide, gabapentin and possibly levetiracetam, may exert their effects via an interaction with voltage-operated calcium channels [11]. The result of Naloxone (3 mg/kg) was administered 15 mins before the Hippocratea welwitschii ethanolic extract. PTZ (85 mg/kg) was injected 30 minutes after the administration of Hippocratea welwitschii In order to elucidate the mechanism involved is shown in Table 3. Values are the mean ± SEM of five mice and show that the extract (200 mg/kg) provided 40% protection (p<0.05) which is significant, student-t-test. In contrast, the mean onset of convulsions in PTZ-induced convulsions was increased with 400 mg/kg of Hippocratea welwitschii extract (Table 3).
Table 3. Mechanistic study of anticonvulsant effect of hippocratea welwitschii against PTZ-induced seizure in mice via GABA-ergicbenzodiazepine mechanism: Flumazenil + H.W. +PTZ (N=5).
| Dose | ID | Wt. in g | Time | Onset of clonus (mins) | Mean onset of clonus (mins) | Hind limb Tonic extension (HLTE) | Mean HLTE (mins) | No. dead | % protection | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| *H.w (200 mg/kg oral)+10 mg/kg flu(IP) +85 mg PTZ(IP) | RS | 18 | 9.34 am | 93 | 76.4 | 480 | D | 40% | |||
| HDLL | 17 | 74 | - | A | |||||||
| HD | 16 | 96 | - | A | |||||||
| HDRS | 19 | 67 | 403 | 222.6 | D | ||||||
| TL | 25 | 52 | 230 | D | |||||||
| Involvement of Opiod receptor using naloxone | |||||||||||
| Nal 3mg/kg+H.w (200 mg/kg) + PTZ | RSLS | 20 | 10.00 am | 41 | 62 | 181 | D | 20% | |||
| BKSTRP | 22 | 62 | 149 | D | |||||||
| HDBK | 20 | 96 | 96 | D | |||||||
| BKRS | 19 | 49 | 147 | 114.6 | D | ||||||
| LS | 20 | 62 | - | A | |||||||
| 10 mg/kg flu+3 mg/kg diazepam+85 mg/kg PTZ | RLLA | 20 | 10.23 am | 38 | 40.8 | 69 | D | 0% | |||
| RALS | 19 | 41 | 72 | D | |||||||
| LLLA | 16 | 44 | 55 | 67.2 | D | ||||||
| RSLS | 18 | 40 | 72 | D | |||||||
| TL | 20 | 41 | 68 | D | |||||||
| Flu=flumazenil, PTZ=Pentylenetetrazole, Nal.=Naloxone | |||||||||||
| p<0.05 (Student t-test). | |||||||||||
The ability of the extract (Hippocratea welwitschii) to exhibit activity against these two types of seizures suggests that it may act through different mechanisms to elicit its anticonvulsant effects, such as voltage-gated sodium, calcium, and potassium or GABAergic pathway. The fact that flumazenil reduced seizure onset and attenuated the protection against seizure provided by Hippocratea welwitschii suggest that the extract may contain substance(s) that interacts with the benzodiazepine site in the GABAA-benzodiazepine receptor complex.
Effect of naloxone on the anticonvulsant activity of alcoholic root extract of Hippocratea welwitschii in Pentylenetetrazole (PTZ)-induced seizure in mice:
Diazepam (3 mg/kg) body weight increased the mean onset of convulsion and offered 60% protection (p<0.05). Flumazenil (10 mg/kg) reduced the onset of convulsion in diazepam-treated mice as shown in Table 3 and reduced the protection against seizure from 40%-0%. Similarly, the same dose of flumazenil reduced the onset of seizure in Hippocratea welwitschii extract-treated mice from 0%-40% (p<0.05). In addition, flumazenil completely blocked the protection provided against seizure (Table 3). However, the protection against seizure in Hippocratea welwitschii extract-treated mice was 20% in the presence of naloxone (Table 3). These observations are corroborated by studies on the mechanism of action of AEDs already in use [11]. It has also been reported that AEDs effective in the therapy of generalized seizures of (absence or myoclonic) petit mal type epilepsies exhibit dose-dependent suppression of various seizure patterns induced by PTZ PTZ-induced seizures are similar to the symptoms observed in the absence seizures and drugs such as valproate and ethosuximide which are useful in the management of absence seizures inhibit PTZ-induced seizures [12,13]. At the cellular level, one of the basic mechanisms of actions of AEDs such as diazepam and valproate is the suppression of T-type calcium currents in thalamic neurons [14-16].
The results of the study have demonstrated that Hippocratea welwitschii is sedative and possesses biphasic anticonvulsant activity on the animal models used in the investigation and this provides a rationale for its use in traditional medicine for the treatment and management of epilepsy by communities over the years. The biphasic activity observed in PTZ-induced studies may probably be due to possible interactions among constituents of the crude extract.
Conflict of Interest
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Citation: Okoh-Esene R.U. et al. The Evaluation of Sedative, Anti-Convulsant Properties and the Mechanism of the Action Pathway of the Ethanolic Root Extract of Hippocrateae welwitschii. Nat Prod Chem Res. 2023 11(3), 15-21.
Received: 10-Jul-2023, Manuscript No. npcr-23-25780; Editor assigned: 12-Jul-2023, Pre QC No. npcr-23-25780(PQ); Reviewed: 17-Jul-2023, QC No. npcr-23-25780(Q); Revised: 23-Jul-2023, Manuscript No. npcr-23-25780(R); Published: 30-Jul-2023, DOI: 10.35248/2329-6836.23.11.3.15-21
Copyright: ©2023 Okoh-Esene R.U. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.