Research Article - (2021) Volume 9, Issue 6
Marine natural products have attracted the attention of biologists and chemists the world over for the past five decades. As a result of the potential for new drug discovery, scientists from different disciplines, such as organic chemistry, bioorganic chemistry, pharmacology, biology and ecology have become interested in marine natural products. This interest has led to the discovery of almost 8,500 marine natural products to date and many of the compounds have shown very promising biological activity. The ocean is considered to be a great source of potential drugs. The chemical compounds, which are isolated from marine sources usually consists of nitrogen containing heterocyclic rings. Several sydnone derivatives show a broad range of physiological activities, including antimicrobial, anti-inflammatory, analgesic and antipyretic properties. Hence, chemists have been enthusiastically pursuing the synthesis of such derivatives.
casinoplus vdcasino betriyal betriyal betriyal betriyal betriyal betriyal betriyal betriyal betriyal casinoplus casinoplus casinoplus maltcasino almanbahis melbet betsat fenomenbet betmatik
Marine natural product,Potential drugs, Thiazoles, Halomethyl compound, Sydnonyl moiety.
Thiazoles and their derivatives exhibit various biological activities such as antimicrobial, anti-inflammatory, antiviral, antituberculosis and cytotoxic activities, among othersConsequent to these reports, the present study seeks to synthesize a series of novel thiazole derivatives that contain the sydnonyl moiety, with the aim of obtaining new biologically active compounds. In view of the continued interest in the development of simpler and more convenient synthetic routes for preparing heterocyclic systems, an efficient and useful method is reported herein to synthesize some new sydnone-substituted thiazoles.
1. To Synthesis 4-amino-2-arylamino-5-(3-arylsydnon-4-oyl)thiazoles. 2. To characterize these newly synthesized thiazoles by various spectral analysis.
Synthesis of 4-amino-2-arylamino-5-(3-arylsydnon-4-oyl)thiazoles
1. Synthetic strategy and planning: Based on the long-standing interest in the synthesis of 2-aminothiazoles, I have conceived the following retro synthetic strategy for the access of diaminothiazoloylsydnones [1] (Figure1). In the above scheme, the leaving group LG could be either -NH2 or as we had found some time ago, it could be a O2NNH- group as well. We decided to examine both groups as leaving group LG in the above scheme (Figure 2). Accordingly, the required thiourea derivative would provide the [C4-N3-C2-S1] atoms that go into the making of the thiazole ring. The remaining C5 atom would originate from an α-haloketone where R2 would be sydnonoyl. Thus, out of the four N atoms in the amidinothiourea derivative, where X = H or NO2, three are incorporated into the product.
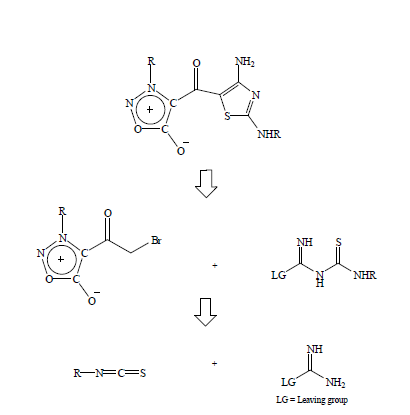
Figure 1: Retro synthetic strategy for the access of diaminothiazoloylsydnones.
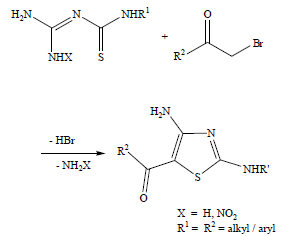
Figure 2: Synthesis of 4-amino-2-arylamino-5-(3-arylsydnon-4-oyl)thiazoles.
2. Synthesis of Precursors:
A. Synthesis of 4-bromoacetyl-3-arylsydnones
The synthesis of the halomethyl compound required for the present [4+1] thiazole ring assembly namely 3-aryl-4-bromoacetylsydnonewas synthesized in a six step process (Figure 3).
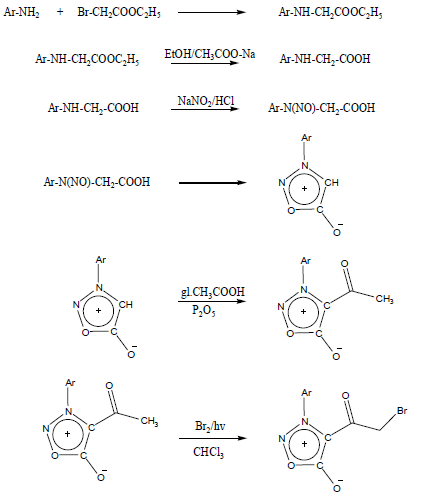
Figure 3: Synthesis of 4-bromoacetyl-3-arylsydnones.
n brief, starting from arylamine and ethyl bromoacetate, the ethyl ester of N-arylglycinewas prepared. This on hydrolysis gave N-arylglycine which upon nitrosation yielded N-nitroso-N-arylglycine 27 which was cyclised next to obtain 3-arylsydnone . In the next step, acetylation of 3-arylsydnone gave 4-acetyl-3-arylsydnone, subsequent bromination of which provided the required 3-aryl- 4-bromoacetylsydnone. Reported procedures from literature which has been suitably modified for our laboratory conditions were used in these reaction steps [2-3].
B. Synthesis of amidinothioureas Nitroguanidine, which was prepared by the isomerisation of guanidine nitrate on treatment with aryl isothiocyanates in the presence of a base gave 1-aryl-3-(N-nitroamidino)thiourea1-Amidino- 3-arylthioureas (31) were prepared by the reaction of aryl isothiocyanates with guanidine carbonate in the presence of sodium hydroxide [4].
C. Synthesis of 4-amino-2-arylamino-5-sydnon-4-oylthiazoles (16) To a solution of 1-aryl-3-(N-nitroamidino)thioureain N,N-dimethylformamide (DMF), 3-aryl-4-bromoacetylsydnone was added followed by triethylamine (Et3N). The thin layer chromatogram of the crude product showed a fluorescent yellow spot as the only significant product. As a representative example, the reaction of 4-bromoacetyl-3-phenylsydnonewith 1-(N-nitroamidino)-3-phenylthioureais described below in detail. The reaction afforded an orange crystalline substance.
D. Elemental Analysis Based on elemental analysis, the molecular composition of the compound was found to be C18H13N5O3S. The IR (KBr) spectrum of the compoundshows peaks at 3362, 3277 and 3070 cm-1 which have been assigned to N-H vibration of amino groups. The IR spectrum further shows a strong peak at 1741 cm-1, which is attributed the C=O group in sydnone. The stretching band of the highly conjugated carbonyl group occurs at 1601 cm-1. These assignments are supported by the observation of a νC=O of a sydnone carbonyl group at 1781 cm-1 and a νC=O bandarising from a highly conjugated pentadienone carbonyl at 1644 cm-1 in the case of 5-phenyl-1-(3-phenylsydnon-4-yl)-penta-2,4-dien-1-one as reported recently by Sanyal and Badami. The presence of a phenyl substituentis indicated by the peaks at 754 and 688 cm-1 arising fromthe νC-H bending bands of phenyl ring hydrogens (Figure 4). The 1H NMR spectrum (300 MHz) shows a broad peak at 9.18 ppm due to the –NH group. The aromatic region shows a set of three multiplets together accounting for ten aryl hydrogens. These multiplets are seen at 7.18-7.26, 7.39-7.49 and 7.56-7.65 ppm. The FAB MS shows a strong [M+H]+ peak at m/z 380, which confirms the molecular mass of the compound to be 379 in accordance with the elemental analysis data. The 13C NMR spectrum of the compound shows ten peaks, four of which appear to arise from two carbons each, thus accounting eighteen carbon atoms. The peak at ν 170.76 ppm is assigned to the carbonyl carbon of the sydnone moiety. This assignment is based on a similar observation in the case of 5-phenyl-1-(3-phenylsydnon- 4-yl)penta-2,4-dien-1-one where the sydnonyl carbonyl carbon was seen at ν 174.55 ppm, as reported by Sanyal and Badami. (Sanyal and Badami, 2009) Based on these data the compound is formulated as 4-amino-2-phenylamino-5-(3-phenylsydnon-4-oyl)thiazole [5-6] (Table 1).
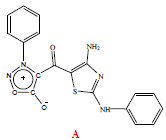
Figure 4: Synthesis of 4-amino-2-arylamino-5-sydnon-4-oylthiazoles
| A | Ar | Ar1 |
|---|---|---|
| Phenyl | Phenyl | |
| 4-methylphenyl | ||
| 4-methoxyphenyl | ||
| 4-chlorophenyl | ||
| Phenyl | ||
| Â | 4-methylphenyl | 4-methylphenyl |
| 4-methoxyphenyl | ||
| 4-chlorophenyl | ||
| Phenyl | ||
| Â | 4-methoxyphenyl | 4-methylphenyl |
| 4-methoxyphenyl | ||
| 4-chlorophenyl | ||
| Phenyl | ||
| Â | 4-chlorophenyl | 4-methylphenyl |
| 4-methoxyphenyl | ||
| 4-chlorophenyl |
Table 1: Synthesized 4-amino-2-arylamino-5-(3-arylsydnon-4-oyl)thiazoles 16a-p.
From amidinothioureas, we have synthesized fifteen novel 4-amino- 2-arylamino-5-(3-arylsydnon-4-oyl)thiazoles A1-15 in 80-85% yield andhave characterized all the synthesized 2-amino-5-(3-arylsydnon- 4-oyl)thiazoles by various spectral data.
Received: 11-Jun-2021 Published: 07-Jul-2021, DOI: 10.35248/2329-6836.21.9.410
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.