Research Article - (2021) Volume 12, Issue 3
Background: Type I diabetes mellitus affects the quality of life of children. Measuring the quality of life of such patients would be important for evaluating treatment outcomes and decision making.
Objective: the aim of this study is to assess the quality of life of children with type I diabetes mellitus attending the pediatric endocrine clinic of a tertiary hospital.
Patients and Methods: This study is a hospital-based descriptive cross-sectional study conducted between April 31 and July 1, 2019. Data were collected from patients and their guardians using the Pediatrics Quality of Life (PedsQL) 4.0 generic core scales and Pediatrics Quality of Life (PedsQL) 3.2 diabetes module. Data were statistically analyzed using the SPSS package. Results described in tables and discussed.
Result: Out of 237 children 120 (50.6%) were females and the age range was between 5-17 years with a mean age of 11.3 years. The age of children at initial diagnosis ranges from 3 months to 15 years with a mean age of 5.11 years. Low family income [AOR 3.05(CI=1.34-6.95)], diagnosis of diabetes less than 1 year [AOR 1.96(CI=1.13-3.39)] and High HgA1C level (≥ 8) [AOR 2.1(CI= 1.01-4.40] have association with poor quality of life.
Conclusion: Type I DM affects the quality of life of children. Assessment is important for further intervention and planning.
Quality of life; Type I DM; Pediatrics; Tikur Anbessa Hospital; Addis Ababa
The commonest endocrine-metabolic abnormality that affects children and adolescents is Type I diabetes mellitus (T1DM). It has a great negative impact on the physical and emotional development of affected individuals [1-4].
Out of 15 million diabetic populations worldwide, T1DM accounts for approximately 10%, and this is over 90% of childhood and adolescent diabetes. Males and females are almost equally affected. There are two peaks at presentations; 5-7 years and at the time of puberty [1,2]. A hospital-based study in school children in Addis Ababa showed a prevalence rate of 2.81/1000 children [5].
According to the World Health Organization definition, quality of life is the way of perceiving his/her position in the system of culture and values. Health-related quality of life implies the magnitude in which any medical condition affects the daily physical, emotional, mental, and contextual well-being of an individual. It is the subjective perception of own health. This concept is therefore increasingly considered as a relevant ‘patient-reported outcome’. Health-related quality of life (HRQoL) measures help to evaluate different aspects of well being and functioning of the individual. In recent years, HRQoL has become a relevant treatment outcome measure from epidemiological and clinical perspectives [3,5-12].
Measuring QoL of children affected with type I DM is indispensable for evaluating results of treatment, consequences of this chronic disorder, and for decision-making in healthcare. In the course of assessing QoL among children with diabetes, PedsQL 4.0 generic core scales and PedsQL 3.2 diabetes module are applied. Both tools are validated in many studies [13-19].
The Pediatrics Quality of Life 3.2 Diabetes Module (PedsQL 3.2) is diabetes-specific HRQoL module that contains 33 items in 5 dimensions. The five dimensions are diabetic symptoms (15 items), treatment barriers (5 items), treatment adherence (5 items), worry (3 items), and communication (5 items). The results are recorded on a 5-point Likert scale from 0 (never) to 4 (almost always). The items are reverse scored and linearly transformed to 0-100 scale (0=100, 1=75, 2=50, 3=25, 4=0). A higher score indicates better HRQoL [13-19].
PedsQLTM 4.0 generic core scales contain questionnaires to be filled by the parents and children, contains 23 items comprising of 4 dimensions (physical functioning, emotional functioning, social functioning, school functioning). Measurement is done on a 5-point Likert scale from 0 (never) to 4 (almost always) and scores were transformed on a scale from 0-100. A higher score indicates better HRQoL [13-19].
There is no published study on the quality of life of children with type 1 DM in Ethiopia. Therefore, this study is done to assess the General QOL and HRQoL in pediatric patients attending the diabetic follow up clinic of Tikur Anbessa Specialized Hospital using the Amharic version of both tools and investigate the associated factors affecting the quality of life of children with T1DM.
Patients and Methods
Study setting
The study was conducted in Tikur Anbessa Specialized Hospital (TASH); Addis Ababa, Ethiopia. Addis Ababa is the capital city of Ethiopia and located at 2355 meters above sea level with a population of 2,739,551 inhabitants. It is the largest referral hospital in Ethiopia with many specialties and sub-specialties and is the teaching hospital of Addis Ababa University, College of Health Sciences. TASH has many departments among which pediatrics and child health is one of them. It has around 800 beds and serves nearly 350,000 patients as outpatients and inpatients annually.
The endocrine clinic is an outpatient follow up unit of pediatric patients with various endocrine disorders. The clinic works 2 days per week on Wednesday and Thursday and DM patients are seen on Thursday. It is run by pediatric endocrinologists, pediatric and child health residents, and trained nurses. Children under the age of 18 years attend the clinic. Currently, around 200 patients attend the clinic per month and among them, around 180 of them are T1DM patients and around 40-50 patients are seen per clinic day.
Five nurses were trained per the protocol as data collectors. Scoring of the QoL in the questionnaire was done using both the PedsQLTM 4.0 generic score scales and PedQLTM 3.2 diabetic module. This scoring system was explained in simple terms to the patients and caregivers by the data collectors so that participants could understand.
Study participants
Children and teenagers account for the majority attending the pediatric endocrine clinic. This group of children is accompanied by their parents or caretakers. The source population for the study were primary caretakers of children under the age of 15 years attending the clinic and also children who were able to give information.
Sample size determination
The sample size was calculated using a single population proportion formula with 95% confidence limits and >0.05 as statistical significance. Taking a 10% rate of non-responders the final sample size was 422. Taking sample size correction for a finite population whose sample sizes proportion greater than 5% of the source population which is approximately 500, the sample size with additional correction of 10 % non-responders was 238.
Sampling technique, inclusion and exclusion criteria
A convenience sampling technique was used. Inclusion criteria include primary caregivers of children who have T1DM and patients who are under the age of 17 years attending the TASH pediatric endocrine clinic and primary caregivers who have cared for the child for more than 6 months.
Exclusion criteria include primary caregivers who didn’t give consent, children who had comorbid chronic medical illness, and who were too ill to participate.
Data management and data analysis
Data cleaning and analysis was done using SPSS version 21 software. Descriptive analytical statistics were used as applicable.A statistically significant association was taken.
Data collection
Piloting of the questionnaire was done in a sample of five children attending the follow-up clinic. These children weren’t included in the study. Findings from the pilot were utilized to modify any questions on the standard questionnaire that require further adjustment to be better suited for our community’s cultural and social standards.
Data collection protocol was prepared and a face-to-face interview was conducted. Data were collected on socio-demographic characteristics, health-related characteristics, and psychosocial conditions of participants and their children.
During the data collection, regular and periodic supervision was made for quality and completeness by the primary investigator.
Ethical consideration
Ethical clearance was obtained from the Department of Pediatrics and Child Health, College of Health Sciences, Addis Ababa University. Written consent was obtained from guardians and. Participant confidentiality was assured.
A total of 237 children with Type I DM were eligible for the study and 156 (65.8%) of the children were brought to the clinic by their mothers, while 69 (29.1%) of them were brought by their fathers. The remaining came with primary caretakers other than their parents. The age of children in the study ranges from 5-17 years, with mean age of 11.43 (SD ± 3.65) years and 159 children (67.1%) were above 10 years of age. Age at initial diagnosis of the illness ranges from 3 months to 15 years with a mean age of 5.11 years (SD ± 3.2).The socio-demographic characteristics of participants is as shown in Table 1.

Based on the BMI for age of children, 5.5% were overweight (between +1 Z score and +2 Z score), 3.8% were obese (>+2 Z score), and 6.3% severely wasted (<-3 Z score). The nutritional status of children is shown (Figure 1).
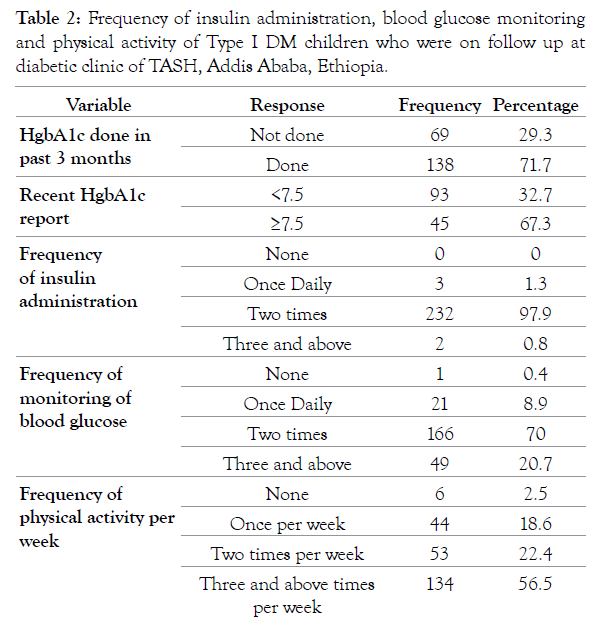
Only 138 (71.7%) of the participants had a recent hemoglobin A1C result with the mean of 8.9 (SD 2.03) and ranging from 5.6 to 14.4. Out of these, 67.3% of the children had a HgA1C level greater than or equal to 7.5, and the remaining have less than 7.5 (Table 2).
As shown in Table 3, the QoL scores were lower for emotional (76.5 ± 18.1) and School (81.3 ± 17.2) domains as compared to other generic score scales. In the Diabetes Module QoL score for worry (67.98 ± 26.67) was found to be low.
| PedsQL- Generic Core Scale (mean ± SD) | ||
|---|---|---|
| Physical | 88.7 ± 14.4 | |
| Emotional | 76.5 ± 18.1 | |
| Social | 90.3 ± 13.5 | |
| School | 81.3 ± 17.2 | |
| Total | 84.2 ± 6.45 | |
| PedsQL- Diabetes Module (mean ± SD) | ||
| Diabetes symptoms | 80.65 ± 16.18 | |
| Treatment barriers | 83.2 ± 16.56 | |
| Treatment adherence | 86.96 ± 15.86 | |
| Worry | 67.98 ± 26.67 | |
| Communication | 88.87 ± 16.72 | |
| Total | 81.53 ± 8.22 |
Table 3: Summary of the results of the PedsQL Generic core scale and PedsQL Module for children with type I DM and their parents who were on follow up at TASH, Addis Ababa, Ethiopia.
Considering only the Generic Core scale and taking 75 as a cut of point, 16.8% of them have a poor quality of life from which 45% of them are females. Also, from the Diabetes module, 37.7% of the participants have poor QoL results, whereby females account 56.9%.
The total mean QoL result of the study was 82.72 (SD10.9). Taking 75% as a cut of point for good quality of life 21.5% of the participants had poor quality of life from which 45.1% are male participants.
Female children had a higher percentage of good QoL than males in the total QoL result. However, when subscale scores were analyzed, girls have poor scores regarding the Diabetes QoL module score.
In this study, lower family income [AOR 3.05(CI = 1.34-6.95)], less than one-year duration since diagnosis of DM [AOR 1.96(CI= 1.13-3.39)] and High HgA1C level (≥8) [AOR 2.1(CI= 1.01-4.40] have a strong association with poor Total QoL (Table 4).
| P-Value | COR: 95%CI | P-Value | AOR: 95%CI | |
|---|---|---|---|---|
| Marital status | ||||
| Married | 1.0 | |||
| Single parent | 1.43(0.66-3.1) | |||
| Mother’s educational status | ||||
| Illiterate | 1.00 | |||
| Complete primary education | 0.104 | 1.35(0.68-2.71) | ||
| Complete secondary education | 0.090 | 1.15(0.63-2.08) | ||
| Complete college diploma, degree and above | 0.193 | 1.04(0.57-1.92 | ||
| Father’s educational status | ||||
| Illiterate | 1.00 | |||
| Complete primary education | 0.146 | 1.49(0.83-2.66) | ||
| Complete secondary education | 0.248 | 1.14(0.68-1.9) | ||
| Complete college diploma, degree and above | 0.321 | 1.02(0 .5-2.1) | ||
| Family income | ||||
| <1000 birr | 0.0001 | 2.08(1.13-3.83) | ||
| 1000- 5000 birr | 0.0031 | 2.17(1.27-3.69) | 0.0001 | 3.05(1.34-6.95) |
| 5000- 10,000 birr | 0.132 | 0.2(0.12-0.33) | ||
| >10,000 birr | 1.00 | |||
| Age of the child | ||||
| <10years | 0.482 | 1.61(0.97-2.68) | ||
| >10years | 1.00 | |||
| Sex | ||||
| Male | 1.00 | |||
| Female | 0.758 | 0.36(0.7-1.76) | ||
| Duration since the diagnosis of DM | ||||
| <1 year | 0.0026 | 1.49(1.03-2.5) | 0.0033 | 1.96(1.13-3.39) |
| 1-5 years | 0.113 | 0.61(0.33-1.11) | ||
| > 5 years | 1.00 | |||
| Frequency of insulin administration | ||||
| None | 1.00 | |||
| Once Daily | 0.065 | 0.78(0.46-1.34) | ||
| Two times | 0.482 | 0.61(0.25-1.51) | ||
| Three and above | 0.863 | 1.7(0.9-3.22) | ||
| Frequency of blood glucose monitoring | ||||
| None | 1.00 | |||
| Once Daily | 0.946 | 1.15(0.63-2.08) | ||
| Two times | 0.842 | 0.8(0.5-1.13) | ||
| Three and above | 0.964 | 1.35(0.68-2.71) | ||
| Frequency of physical activity per week | ||||
| None | 1.00 | |||
| Once per week | 0.881 | 0.61(0.33-1.11 | ||
| Two times per week | 0.425 | 0.92(0.5-1.7) | ||
| Three and above times per week | 0.532 | 1.61(0.97-2.68) | ||
| BMI for age | ||||
| Normal | 1.00 | |||
| Obese | 0.143 | 1.49(0.88-2.5) | ||
| Over weight | 0.090 | 0.37(0.15-1.09) | ||
| Moderate wasting | 0.018 | 1.61(1.22-2.62) | ||
| Sever wasting | 0.062 | 1.49(0.83-2.66) | ||
| HgA1C result | ||||
| <7.5 | 1.00 | |||
| >7.5 | 0.001 | 2.08(1.13-3.83) | 0.001 | 2.1(1.01-4.4) |
Table 4: Statistical analysis of Pediatric quality of life in children with Type I DM and who were on follow up at the diabetic clinic of TASH, Addis Ababa, Ethiopia.
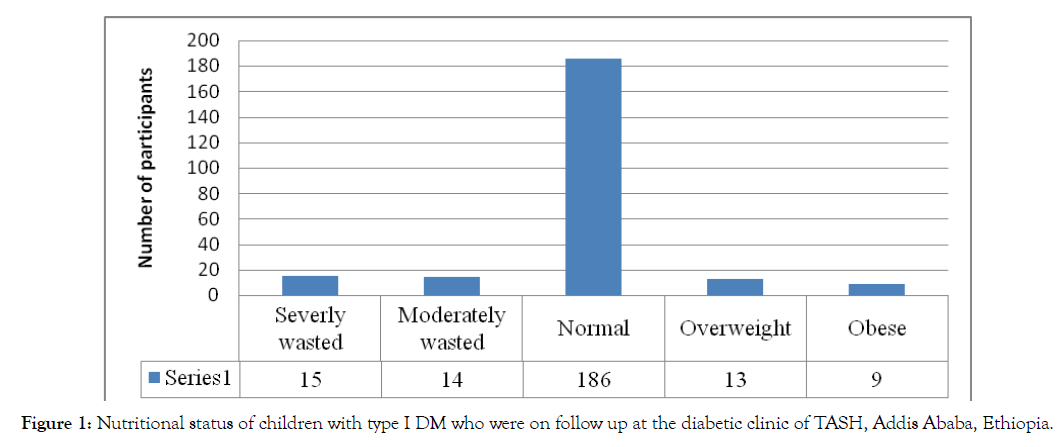
Figure 1: Nutritional status of children with type I DM who were on follow up at the diabetic clinic of TASH, Addis Ababa, Ethiopia.
In our study, the mean total QoL score 82.9, is consistent with studies done by Naughton and colleagues [5] (mean 82.2), as well as Ozyazlcloglu and Avdal [10] (mean 79.91). A study done by Abdul- Rasoul and his colleagues [20] in Kuwait (mean 75.6), as well as another study in Saudi Arabia (mean 60.3), reported lower total QoL score [2].
Children and parents in our study revealed lowest scores on the emotional subscales (mean 76.5) and worry (mean 67.98) which is similar to the results obtained by Naughton and co-workers (mean 73) [5]. Another study done in Saudi Arabia (mean 52.8) [9], as well as the report of Morandini and associates [8], recorded the lowest score on the worry domain. A study done by Kalayvato [21] showed poor emotional HRQoL compared to the healthy controls was also similar to our result. Lack of autonomy, requirement of daily insulin injection and need to control their diet and preoccupation with chronic complications may account for lower emotional and worry scores.
In our study the highest score was found for the social subscale (mean 90.3); other investigators also reported to be highest among all the domains of the Peds QoL. Al Buhairan and co-workers [9] reported family relationships and social functioning (80.9%) is highest also Ozyazlcloglu and Avdal [10], Abdul-Rasoul and coworkers [20], as well Kalayvato and colleagues [21] reported high social functioning score.
The physical subscale in our study was also high (mean 88.7), but Naughton and colleagues [5] reported low physical functioning (mean79), which is next to the emotional score. Likewise, Abdul- Rasoul and co-workers [20] and Kalayvato and associates [21] reported a lower score for the physical functioning subscale that is in contrast to our finding. This could be associated with the difference in lifestyle that children in our set up have better adaptation.
In our study females have a higher percentage of total QoL (55%) than males. However, when subscale scores were analyzed, girls revealed poor scores regarding the Diabetes QoL module score (56%). Similarly, Naughton and associates [5] reported a significant age and sex interaction with HRQoL, such that in older groups recorded scores were lower for girls but higher for boys. Anderson and co-workers [12], Abdul-Rasoul and co-workers [20], as well as Lukacs and colleagues [22] reported girls to have lower QoL than boys. In contrast to these, Basl and associates [11] reported no gender-related change in the QoL from their study. The reason for girls to have more diabetes-related poor scores could be associated with their tendency to attain puberty and mature earlier than boys.
The most important finding of our study is that a high HgA1C level [AOR 2.1(CI= 1.01-4.40), p-value 0.001] has a significant association with poor HRQoL. Similarly, Anderson and associates [12] reported that HRQoL was significantly related to HbA1c (P<0.001). Several other investigators [7, 8, 10, 23, 24] also reported that quality of life is inversely related to the HgbA1c level. However, Naughton and colleagues [5] have reported that the HbA1c level was not significantly associated with HRQoL. Although a cause-and-effect relationship cannot be determined, the association between HbA1c and QoL is so consistent that it is now justified to consider QoL and metabolic control to be equally important in the management of T1DM. Whether good metabolic control increases QoL or high QoL enhances metabolic control or both, is an area that requires further investigation.
In this study, the duration of illness was an important predictor of QoL in the diabetes module, since an increase in the duration of illness is associated with an improved score. In accordance with this, Abdul-Rasoul and co-workers [20] have reported that total QoL scores increased with age throughout the age groups and increased duration of diabetes. Likewise, Naughton and colleagues [5] reported that youth with longer diabetes duration reveals better functioning. Conversely, several other investigators [6,7,11,20] reported a lack of correlation between the duration of illness and HRQoL. The presence of association observed in our study could be explained by the fact that adolescents with longer duration of illness will have better knowledge about the illness than those with short duration of illness, and hence they will manage their disease more subjectively and independently.
In our study, lower family income was highly associated with poor QoL [AOR 3.05(CI=1.34-6.95); p-value 0.0001]. Conversely, Laffel, and co-workers [6] reported a lack of significant correlation between QoL and the family’s socioeconomic status. The observed high association of low family income with poor QoL in our study reveals the strong dependence of providing good medication, food, etc. with good family income, as there is no other means for the family to cover the costs associated with diabetes. In other words, Ethiopian families are always expected to cover their medical bills, school fee, and the likes from their pockets.
No significant correlations were observed in this study between the total quality of life reported by parents with the child’s age, BMI, number of daily injections, total daily insulin dosage, frequency of blood glucose monitoring, frequency of exercise, education level of the parents and single-parent families. This finding is consistent with the research done by Naughton and co-workers [5] that showed no significant association between HRQoL and demographic variables, BMI, and comorbid conditions. Ausili and Tabacco [21- 26] also reported the absence of a significant correlation between the number of daily injections of insulin, insulin-units/kg, BMI, hypoglycemia symptoms, and HRQoL. Laffel and co-workers [6] also reported a lack of significant correlations between QoL with their age, education level of parents, and the number of daily injections, total daily insulin dosage, or frequency of blood glucose monitoring. In another study, Cobuz and associates [26] have also reported absence of significant correlation regarding QoL and the age of the child, type of family, number of daily injections or the total daily dose of insulin (units/ kg); however, they have observed significant correlation between QoL with physical activities and BMI. In contrast to these, Anderson and his associates [12] have reported that level of parental education, frequency of blood glucose monitoring, and physical activity to be significantly related to HRQoL.
Type I DM affects the quality of life of children and diabetic management should include assessment of HRQoL.
We would like to thank the Department of Pediatrics and Child Health for allowing us to conduct the study.
Conflict of Interests
We declare no conflict of interest.
Citation: Wondyfraw B, Shimelis D, Yeshiwas S (2021) Quality of Life of Children with Type I Diabetes Mellitus Attending Follow Up Clinic at Pediatric Endocrine Clinic of a Tertiary Hospital in Addis Ababa. J Diabetes Metab 12:867.
Received: 26-Oct-2020 Published: 30-Mar-2021, DOI: 10.35248/2155-6156.21.12.871
Copyright: © 2021 Wondyfraw B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.