Research Article - (2024) Volume 15, Issue 3
An elevated risk of mortality, secondary sarcopenia, multiorgan dysfunction, sepsis, and severe oxidative stress are all brought on by major burn injuries. They also set off a systemic inflammatory response and a chronic hypermetabolic and hypercatabolic state. Following heat damage, poor clinical outcomes have been linked to calorie shortfalls, negative protein balance, and antioxidant micronutrient insufficiency. In this situation, early enteral feeding at the onset of resuscitation, together with tailored nutrition therapy, is recommended. Various pharmacological and dietary therapies targeting immunological and metabolic response modulation have been studied during the past 40 years. In this patient population, these tactics have demonstrated the ability to reduce acute malnutrition, control the immunoinflammatory response, and enhance pertinent clinical outcomes. Summarising the most recent research on nutrition therapy and metabolic response in critically ill burn patients is the aim of this updated review.
Burn wounds; Emergency medical care; Intense metabolism; Acrometalization; Tiny nutrition; Phospholipid
Critically sick burn patients represent a model of trauma patients with an early onset of severe hypercatabolism and hypermetabolism, with an energy expenditure (EE) that can be replicated at rest. Even months after the initial thermal aggressiveness, these changes may persist. One A direct correlation exists between the extent of the total body surface area burned (TBSAB) and several biomolecular changes that determine the magnitude of this state [1]. However, the loss of micronutrients (vitamins and trace elements) and macronutrients (proteins) through burned areas is largely caused by heat degradation. Acute starvation, secondary sarcopenia, and acquired muscle weakness are the results of the severe hypermetabolic and hypercatabolic response, which promotes the emergence of infections, multiple organ dysfunction, sepsis, and ultimately death. The systemic inflammatory response, mitochondrial dysfunction, and metabolic changes previously indicated are maintained and perpetuated by oxidative stress, which is facilitated by the negative balance of antioxidant micronutrients over the thermal year [2].
In 2015, [2] Czapran et al. released the outcomes of severely sick burn patients who were included in the 2007–2011 International Nutrition Survey and needed mechanical breathing for more than 72 hours. Based on the analysis of these data, it was determined that the 90 patients had an estimated deficit in expenditure (943±654kcal/d) and an estimated deficit in protein (49±41g/d) [2]. However, these results also show that 21% of the patients passed away during the follow-up period, which began about 60 days after the patients were admitted to the intensive care unit (ICU). Deaths from this cause were associated with greater deficiencies in protein (67±42 vs. 44±39g/d, p=0.03) and energy (1.251±742 vs. 861±607kcal/d, p=0.02).
For these reasons, by reducing the acute malnutrition linked to his severe condition and regulating his immunoinflammatory response, we can better aid in the critically ill burned patient's recovery by administering appropriate metabolic and nutritional therapy [3]. As a result, early, sufficient, and customised nutritional therapy has been implemented, and this has improved clinical outcomes, particularly by lowering the rate of infectious complications, reducing hospital stays, and hastening the healing process. Four Because of this, nutritional therapy is the cornerstone of the treatment plan for a critically ill burn patient [4]. It should begin during the early resuscitation period and continue throughout the healing and final rehabilitation phases. In order to better understand the metabolic changes and nutritional strategies that occur in critically ill burn patients, this review will examine current research on these topics, with a focus on topics like protein and calorie targets as well as pharmaco-nutritional and anticatabolic strategies in this population of patients.
Inflammatory response and energetic metabolism in thermal injury
Systemic inflammation is brought on by stress hormones and proinflammatory cytokines (TNF-α, interleukin IL-6, IL-1β, and tumour necrosis factor TNF-α), which result in a marked and long-lasting hypermetabolic response. An elevated basal metabolic rate, cardiac output, myocardial oxygen demand, muscle protein breakdown, and insulin resistance are the characteristics of this response (Figure 1) [5]. An elevated basal metabolic rate (up to 40% above normal levels) and notable modifications to mitochondrial function characterise the hypermetabolic response brought on by thermal aggression. In healthy humans, oxidative phosphorylation accounts for around two-thirds of oxygen consumption, with thermogenesis accounting for the remaining third [5]. In very ill burn victims, this ratio is inverted, with two-thirds of total breathing dissipating as heat and one-third of mitochondrial respiration paired with oxidative phosphorylation. One of the main causes of hypermetabolism and the elevated basal metabolic rate in skeletal muscle is this mismatch in mitochondrial oxidative phosphorylation [6,7]. Thus, even two (2) years after thermal aggression, Porter et al. [8] showed that these individuals had defective control over mitochondrial coupling with over 30% of the TBSAB, which is linked to a marked increase in thermogenesis. A study by Szczesny et al. [9] used an experimental model of thermal aggression to look at what happens to mitochondrial bioenergetics after a burn. They found that basal respiration and ATP turnover slow down over time. The authors of this study showed that the mitochondrial DNA of cardiac striated muscle and pulmonary tissue were damaged in a way that was different from other tissues. Lastly, this group documented that the effects were even more pronounced in the heart and lungs by studying the development of oxidative stress and the infiltration of neutrophils driven by myeloperoxidase activity [9]. Together with the information from the Porter et al. study, these results show that mitochondrial dysfunction could one day be a real therapeutic target for severely sick burn patients [8]. But before preclinical trials are carried out to evaluate the efficacy and safety of these tactics, these results still need to be confirmed.
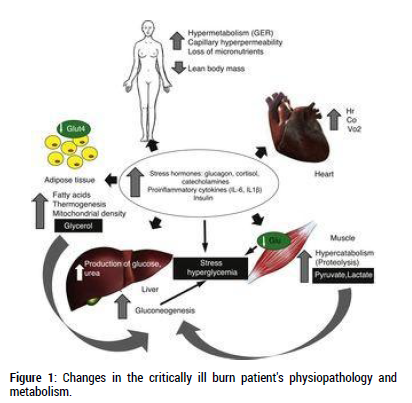
Figure 1: Changes in the critically ill burn patient's physiopathology and metabolism.
Higher levels of IL-6, IL-8, granulocyte-colony stimulating factor, monocyte chemoattractant protein-1, C3 complement, 2-macroglobulin, haptoglobin, 1-acid glycoprotein, and C reactive protein were found in a prospective cohort of 230 critically ill children with over 30% of their TBSAB back in 2014 [10] . This group of patients also had a more intense inflammatory response. In the same way, patients with greater levels of urea, insulin, glycemia, and plasma creatinine had more substantial metabolic changes. These authors ultimately demonstrated that those who did not survive the critical burn had a much greater EE at rest, as determined by indirect calorimetry. There is no doubt that in critically ill burn patients, immunoinflammatory and metabolic changes are predictive of morbidity [10].
To meet the higher caloric needs brought on by the hypermetabolic condition whilst minimising overfeeding is the main objective of dietary guidance in those suffering from burns. The calorie requirements of burned sufferers have been treated and calculated using a variety of methods over the years [10]. The Curreri formula is one of the oldest instances [11]. It was developed by analysing nine individuals and working forwards to estimate the number of calories that were needed to make up for the patients' weight loss when it was first offered in 1972. Many previous models, such as the Curreri formula, exaggerate present metabolism needs; hence, more complex equations with different factors have been developed [10]. Neither of the 46 distinct formulas studied for forecasting caloric requirements in those with burns corresponded well with the observed energy expenditure in 24 individuals, according to the study [12]. Energy consumption does vary after burn, and set formulas frequently result in undernutrition between times when the usage of energy is at its peak then excessively feeding later in the term of surgery.
How glucose is metabolised
During the early stages following the burn, the rate of production and oxidation of glucose and poor tissue extraction from the burn may generate hyperglycemia and hyperlactatemia [11]. Hyperglycemia (values >180 mg/ dl) is associated with adverse clinical outcomes such as hypercatabolism, hypermetabolism, delayed injury healing, a higher incidence rate of infections, and higher mortality. [12,13] The thermal injury induces stress on the endoplasmic reticulum (ER) in the skin and adipose and muscle tissue a phenomenon associated with insulin resistance [14]. This stress on the ER is registered by different types of enzymes, such as the inositol-requiring enzyme 1, the protein kinase RNA-like ER kinase, and the activating transcription factor 6; these proteins are responsible for transcription phenomena, genetic translation, deployment, translocation, and protein secretion. [14,15] Back in 2012, Jeschke et al. [16] showed that the stress markers of ER remain high until 250 days after the aggression, which is consistent with insulin resistance and stress hyperglycemia.
Stoecklin et al. [17] validated this in 229 adult patients who were substantially burned. In these patients, moderate glycemic control (from 100 to 140 mg/dl) with the administration of IV insulin is associated with less glycemic variability and a limited risk of hypoglycemia. Insulin regulates the absorption of glucose in skeletal muscle and adipose tissue while also preventing the production of glucose in the liver. Conversely, insulin influences the generation and discharge of proinflammatory cytokines (TNF-α and IL-1β), anti-inflammatory IL-10, and the expression of adhesion molecules ICAM-1 and E-selectin [18]. In this regard, insulin therapy administered intravenously (IV) to achieve glycemia levels ≤130 mg/dl appears to mitigate hypermetabolic response levels, as 140 mg/dl is linked to decreased rates of morbidity and mortality, as previously proposed by Jetschke et al. [19]. In the context of trauma patients, guidelines advise starting insulin therapy when glycemic levels reach ≥150 mg/dl. This should be adjusted to maintain values <180 mg/dl, particularly <150 mg/dl, thereby preventing hypoglycemia (values <70 mg/dl) [20].
Nevertheless, the notions of diabetes paradox and, specifically, metabolic control have been introduced recently by measuring the levels of glycosylated hemoglobin [21]. As a result of this newfound understanding, the objective range of glycemia in critical conditions will be determined by the value of glycosylated haemoglobin in such a way that levels >6.5–7.0 should make us a little more tolerant of the objective range (160–220 mg/dl); the new objective ranges, however, have not yet been thoroughly studied in critically ill burn patients.
How proteins are metabolised
Serious hypercatabolism results in sarcopenia, which is linked to organic dysfunction, weakening of the muscles, and increased mortality rates [22].
One square metre of burned skin is thought to cause 20–25g of nitrogen loss per day in burned patients, which translates to a 20–25% loss of lean body mass. Similar estimates indicate that, in burned patients not receiving nutritional therapy, the average loss of N is expected to be greater than 0.2g of N/kg/day (15–20 g/day), resulting in a 10% loss of body weight in the first week. Pressure ulcers, immunological dysfunction, changes in scar tissue, and an increased risk of infection are all linked to this acute protein depletion [22].
In other words, a rise of up to 400% in protein synthesis has been documented with the delivery of IV insulin. The hypercatabolic reaction is reduced with the supply of exogenous insulin, altering the balance between synthesis and proteolysis, which essentially occurs in the skeletal muscle. Up to 500% increases in phenylalanine uptake have been reported, suggesting that this insulin effect is mediated by increased amino acid transport [23]. Similarly, nonnutritional strategies like propranolol use increase intracellular free amino acid recycling, which is reused during protein synthesis in the myocyte, and a higher phenylalanine uptake and protein fractional synthesis have been confirmed [19] .
How fats are metabolised
Adipose tissue mobilisation of fatty acids (FA) occurs after the burn. The increase in FA flow towards plasma and lipolysis are both mediated by elevated levels of catecholamines, glycagon, and adrenal corticotropin [24]. The changes in the fatty acid profile become more noticeable between 7 and 10 days following thermal aggression, while lipolysis increases between 5 and 2 months following the aggression. These modifications are favourable to the post-burn inflammatory and immunosuppressive state. However, the analysis of the plasma FA profile reveals a reduction in polyunsaturated FAs with an anti-inflammatory profile and an increase in saturated and monounsaturated FAs with pro-inflammatory action. Additionally, there is a persistent increase in glycerol levels, which translates into greater lipid peroxidation; similarly, most FAs are re-esterified, which is confirmed by the increase of LDL lipoproteins, triglycerides, and the lower level of ketonic bodies. Lastly, we should note that fat infiltration, especially in the liver, which is linked to insulin resistance, is a result of enhanced lipolysis after the burn and raises the morbidity rate [25].
Antioxidant micronutrient status and oxidative stress
With severe depletion of endogenous antioxidant defences from excessive urine excretion and of antioxidant micronutrients like copper (Cu), zinc (Zn), and selenium (Se) through burned areas, burns constitute a model of oxidative stress [26]. This phenomenon is particularly noticeable towards the end of the first week following thermal aggression. Trace element redistribution during the acute phase can also be explained by capillary effusion brought on by systemic inflammation, which also compromises tissue repair mechanisms and lowers antioxidant capacity. The level of 12 trace elements in the exudates of the burned areas was recently measured by Jafari et al. [27] in 15 patients who burned with an average of 29±25% of their TBSAB. Day one saw the greatest losses for the majority of micronutrients, with fewer losses recorded after the first 24 hours [27]. This was especially true for Se, Cu, Zn, boron (B), bromine (Br), iron (Fe), and iodine (I).
These patients also lacked vitamins A, C, D, and E. In light of this, research on vitamin D in various populations of critically ill patients has been very interesting in the past few years [28]. Recently, Blay et al. [29] confirmed severe deficiencies (25-dihydroxyvitamin D<10ng/ml) in n = 46 (14.5%) and insufficiencies (10–29ng/ml) in n = 207 (65.1%) of 318 burned patients, while the remaining 20.4% of patients had normal serum levels of vitamin D. The authors found that patients with vitamin D deficiency or insufficiency had longer stays in the intensive care unit and hospitals [29]. These results, along with the groundbreaking research done by Berger et al. [30,31], have sparked interest in trying to apply the strategy of giving antioxidant micronutrients to critically ill burn patients over the past 30 years.
The course of dietary therapy
When it comes to critically burned patients, the enteral route is the preferred method. The American Burn Association (ABA) [33] and the Spanish Society of Intensive and Critical Care Medicine and Coronary Units, as well as the Spanish Society for Parenteral and Enteral Nutrition (SEMICYUC-SENPE) [34], recommended beginning enteral nutrition as early as possible in 2011. The Inflammation and Host Response to Injury (Glue Grant) [35] recommendations also advocate early enteral nutrition (EN), albeit the best time to initiate EN is never specified. During the first 24 hours, 95% of the 153 burned patients began EN, according to research done in 2011 by Moisier et al. [4] . Less aggressive wound infections (p=0.01) and shorter intensive care unit stays (p=0.03) were linked to early EN. In addition to protecting the integrity of the intestinal barrier, motility, and splanchnic blood flow, some studies have already demonstrated that very early EN during the first 6–12 hours is safe, modulates the hypermetabolic response, significantly lowers levels of catecholamines, cortisol, and glycagon, and increases the production of immunoglobulins. [32,34] Vicic et al.36 also confirmed that an early EN lowers the rate of infections, with the exception of nosocomial pneumonia. Early EN hasn't always improved clinical outcomes for burn patients, nevertheless, according to the actual data that is now available. In order to determine how early EN affected pertinent clinical outcomes in burned patients, such as the injury infection rate, the length of mechanical ventilation, the use of antibiotics, and the mortality rate, Guo et al. [37] carried out a systematic review and one meta-analysis involving six randomised clinical trials (RCT) of critically burned patients back in 2015 [14,15] . The analysis of results did not demonstrate any clinical benefits from early EN in critically sick adult burn patients, even though this study was removed from PubMed and the journal web site at the authors' request.
Regarding the nutritional benefits for burn patients, Sudenis et al. (2015, [38], found that in a cohort of 90 patients with over 10% TBSAB, the effective supply of calories through the enteral route was 19% on day 1 following the burn and 91% on day 14 after the EN was started, on average 9.5 hours after hospital admission [39] .
However, GI dysfunction, intestinal failure brought on by abdominal pressure, and ultimately abdominal compartment syndrome (ACS) are significant factors that may restrict the use of the enteral route in burn patients. ACS can result from paralytic ileus compliance and a reduced abdominal wall due to the energic flow of fluids. Thus, intestinal dysfunction is a contraindication to the enteral route and a sign of parenteral nutrition (PN). Various clinical trials have examined the composition of PN in patients with burns, including the best lipid emulsion to utilise. In a 2005 RCT, García de Lorenzo et al. [40] examined the effects of two different 50/50 emulsions of LCT and MCT (Lipofundin® 20%, B. Braun, Germany) and an olive oil emulsion (ClinOleic® 20%, Baxter, France). The patients were critically ill burn patients. The liver function test was less altered in the patient group that received the olive oil-rich emulsion, but there were no changes in the acute phase parameters, PN tolerability, or fatty acid metabolism. Additionally, in 2008. A retrospective research of two cohorts was carried out by Mateu-de Antonio et al. [41] to examine the effects of a lipid emulsion made entirely of soybean oil (Intralipid® 20%, Fresenius Kabi, Spain) vs an alternate emulsion that contained a lot of olive oil (ClinOleic® 20%, Baxter, Spain). The cohort that received the lipid emulsion rich in olive oil had a high peak value of leukocytes at the end of PN (p=0.036), but there were no differences in the incidence of infection, length of ICU and hospital stays, or mortality rate between the two groups, according to the analysis of the data [41].
Dietary needs
Because of their extreme hypermetabolism and hypercatabolism, critically ill burn patients have high dietary needs. For this reason, the nutritional therapy guidelines focus on increasing the amount of calories and proteins that are available to patients, which improves their chances of survival. As Cunninghan [42], demonstrated in a review of studies that employed indirect calorimetry to quantify basal EE, each patient has a distinct energy supply requirement that depends on their condition after experiencing thermal aggression. For this reason, the EE must be evaluated on a daily and personal basis. The indirect calorimetry process is the preferred method and is currently the accepted standard of reference. Nevertheless, in burning centres, this resource is not always accessible, therefore using prediction equations on a regular basis is necessary. As a result, this method is not advised nowadays. Rimdeika et al. [43] demonstrated that using a single fixed Figure 1 of 25–30 kcal/kg of body weight may result in enteral hyponutrition.
In terms of prediction equation application, the Toronto equation (Table 1) appears to be the most suitable one as it estimates both the variability of energy requirements and the progression of the illness. In children who are critically ill from burns, the Schoffield equation has proven to be the most useful equation [44]. Several studies have demonstrated that EE increases greater during the initial stages of burns, declining gradually thereafter.
| Nutrient | Daily dose suggested | Comment |
|---|---|---|
| Proteins | 1.5 at 2.0g/k/d | Daily supply <20–25% of the total caloric supply. Dose >2.2g/kg/d do not improve the optimal balance of proteins32,34 |
| Lipids | 1.0–1.5g/kg/d | <30% of non-protein calories. Optimize n3/n6 ratio. |
| Carbohydrates | 5–7g/kg/d | Do not exceed 1400–1500kcal/d as carbohydrates. Supply should not exceed 5.0mg/kg/min32,34 and the levels of glycemia 140–180mg/dl should be maintained with IV insulin (in the absence of DM) |
| Glutamine | 0.3–0.5g/kg/d | Should not be administered in the presence of hepatic and renal failure. The definitive recommendation is pending the results from the RE-ENERGIZE study54 |
| Coppera | 4.0–5.0mg | |
| Seleniuma | 300–500µg | Administered as IV sodium selenite or selenious acid |
| Zinca | 25–40mg | |
| Chrome | 15mg/d | |
| Vitamin Ca | 1.0–3–0g/d | First 24h: 66mg/kg/h58 until 110g during the first 24h |
| Vitamin Da | =70 years: 600IU>70 years: 800IU | Vitamin D3 (oral, enteral or parenteral). Vitamin D deficiencies are common (50%) although there is still no definitive recommendation on its supplementation75 |
| Vitamin Aa | 10,000IU | |
| Vitamin Ea | 20–25IU |
Table 1: Nutrient requirements for the critically ill burn patient, including macro and micronutrients in the severally ill burned case.
Use of prediction equations in critically ill adult burned patients
Equations for predicting the energy needs of severely sick burn patients
Toronto equation, which yields findings that resemble indirect calorimetry:
(−4343+ [10.5% BSA]+[0.23calorie intake]+[0.84×Harris Benedict]+[114×rectal temperature in °C]−[4.5×day post-burn]) equals EER.
Equation Carlson
= BME× (0.89142+0.01335×TBSAB) ×m2×AF
Equation Harris-Benedict (multiplying the outcome by the stress factor [1.2– 2.0])
Males age [years] = 66.437 + (5.0033 + 13.7516 * weight [kg])
Females: 655.0955 + 1.8496 height [cm] + 9.5634̗weight [kg] − (4.6756×age [years])
Activity factor of 1.25 (AF), basal metabolic expenditure (BME), body surface area (BSA), square metres of body surface (m2), estimated energy requirements (EER), and total body surface area burned (TBSAB).
Retrospective observational analysis conducted in Back 2010 [45], on a heterogeneous population of critical patients, excluding burned patients, revealed that the mortality rate at 60 days was significantly lower when patients received 80–85% of the estimated caloric target on day 12. More recently, Nicolo et al. [46] confirmed reduced mortality rates when over 80% of the prescribed protein was administered on day 4 (odds ratio [OR]: 0.68; 95% confidence interval [CI]: 0.50–0.91) and day 12 (OR: 0.60; 95% CI: 0.39–0.93) in a group of similar critical patients. It was also confirmed by the authors that patients who received ≥80% of the recommended protein on day 12.46 had a shorter time to hospital discharge (hazard ratio [HR]: 1.25; 95% CI: 1.04– 1.49). This suggests that nutritional adequacy protocols are an effective way to optimise the supply of calories and proteins. A recent study by Conrad et al. [47] in critically ill burned patients revealed that the nursing staff's application of a single nutritional algorithm satisfied 100% of the calories prescribed in 85.4% of the days, as opposed to 61.6% of the days in the retrospective control group on EN. The two groups' ICU stay, mechanical ventilation duration, and death rate, however, did not differ.
Carbohydrate
Carbohydrates (CH) should not exceed 1400–1500 kcal/day as CH34 and should be administered at a dose of 4-5 g/kg/day (Table 1). Rich diets in CH and proteins stimulate the synthesis of proteins and release endogenous insulin, which aids in lean body mass recomposition [48]. However, the only clinical benefit of using enteral formulas low in lipids and high in proteins and CH may be the decreased risk of nosocomial pneumonia in burn patients who have lost at least 10% of their total body surface area.
The recommended dose range for burned patients is 1.5-3.0 times higher than the regular recommended dose, and the dosage should be adjusted according to the total area of the body that has been burned.
Fat
A person's fat content should range from 15–18%, never going above 20–30% of their daily calorie intake that isn't protein (1.0–1.5 g/kg). 34 Omega-6 fatty acids are the main source of fats (proinflammatory impact). Enteral formulae high in antioxidant micronutrients and omega-3 fatty acids, such as γ-linolenic acid and eicosapentaenoic acid, have not yet been examined in burn patients. Nevertheless, in 92 patients with a TBSAB>15% and inhalation injury, Tihista et al.[49] compared the effects of an enteral formula with 50% of the lipids as omega-3 vs. one conventional formula low on fats (18% of energetic supply). Supplementations with omega-3, as the sole immunonutrient strategy, have had positive effects modulating the inflammatory response, promoting better tolerance to glucose, and lowering the risk of infection. According to the data analysis, patients who consume a diet enriched in omega-3 fatty acids have lower rates of sepsis and septic shock episodes and a greater ability to withstand EN.
The proteins
Critically sick burn patients require 50 percent more protein during fasting than do healthy subjects [34] Table 1.Therefore, the protein requirement should not exceed 20–25% of total caloric intake (from >1.5 to 2.0 g/kg/day). It is still unknown, nevertheless, what the ideal protein supply is for these people [50].
Certain nutrients, such as glutamine
Over the past few years, there has been increasing debate about glutamine, an amino acid that is necessary for the treatment of severe conditions. In a group of 80 surgically severely ill patients, Oudemans-van Straaten et al. (2001) demonstrated that plasma glutamine levels <0.42 mmol/l were linked to greater fatality rates. However, in the wake of two or more organic dysfunctions, Heyland et al. [51] were unable to substantiate the glutamine deficit.
One international, multicenter RCT (n = 1.223) conducted in 2013 examined the effects of glutamine and antioxidants using a 2×2 factorial design [51], patients who were given glutamine supplements were given 0.35 g/kg/day (Dipeptiven®, Fresenius Kabi, Germany) via the parenteral route and an additional 30 g/day orally. High dosages of glutamine were found to increase hospital mortality when organic dysfunction was present, according to the examination of the data. In contrast to an enteral diet high in protein, Van Zanten et al. [52] recently gave 300 medical and surgically critically ill patients a diet high in glutamine, omega-3 fatty acids, and antioxidants. A greater 6-month death rate (53.7% vs. 34.5% in the control group, p = 0.044) was linked to a glutamine-rich diet in the severely sick patients.
The use of glutamine dipeptides in critically ill burn patients has been the subject of very few RCTs to date. Following the publication of four RCTs (n = 155) in 2013, Lin et al. [53], demonstrated that glutamine administration may be linked to a decreased rate of hospital mortality (OR = 0.13, 95% CI: 0.03-0.51, p = 0.004) and gramme-negative bacteremia (OR = 0.27, 95% CI: 0.08–0.92, = 0.04). Current research, after incorporating 6 RCTs (n = 225) by Zanten et al. [54], demonstrated a significant reduction in hospital stays (weighted mean difference, expressed in days: 6.06, 95% CI: 9.91-2.20, p = 0.002) and a positive effect of enteral glutamine administration on the overall survival rate (risk ratio [RR]: 0.22, 95% CI: 0.07–0.62, p = 0.005), although no effect on nosocomial infections was established.
Heyland et al. [55] are currently conducting the pragmatic, double-blind, multicenter randomised trial of enteral glutamine to minimise thermal injury (RE-ENERGISE, NCT00985205), which will involve 2700 critically ill burn patients receiving either enteral glutamine (0.5 g/kg/d) or a placebo. Hospital survival is the main objective of this randomised controlled trial (RCT), which is being carried out in more than 60 burned centres across North America, Europe, and South America. Secondary objectives include the incidence of nosocomial bacteremia caused by gramme-negative bacteria, the length of hospital and intensive care unit stays, and the quality of life following the burn. It anticipates results by 2021.
Micronutrients rich in antioxidants: antioxidant cocktails
Giving antioxidant mixtures to burn patients may lower the risk of infection complications, according to the groundbreaking research done in this regard by a Swiss group under the direction of Berger [30,31]. Therefore, in 2006, Berger et al. [30] validated, after adding 2 RCTs (n = 41), that giving Cu 2.5 at 3.1 mg/d, Se 315 at 380 μg/d, and Zn 26.2 at 31.4 mg/d for 8–21 days decreased the incidence of nosocomial pneumonia. Subsequently, the same group demonstrated that parenteral administration of a single antioxidant cocktail containing Cu, Se, and Zn over 14-21 days decreased the incidence of pneumonia and enhanced antioxidant status as well as skin and plasma levels of Se and Zn. 31
Hospital stays were decreased in 2015 by Kurmis et al. [56], when parenteral administration of Cu and Se as well as enteral and parenteral administration of Zn were combined. Nonetheless, there is ongoing debate on the real data regarding the impact of antioxidant micronutrient supplementation on the antioxidant status of critically ill burn patients. To that end, Raposio et al. [57], recently demonstrated in a small RCT (n = 20) that enteral treatment of squalene 100 mg, vitamin C 30 mg, coenzyme Q10 10 mg, zinc 5 mg, β-carotene 3.6 mg, bioflavonoid 30 mg, and selenium 55 μg for two (2) weeks did not enhance the antioxidant capacity of plasma. Antioxidant therapy length varies according to TBSAB; in fact, 20–40% of TBSAB require a 7-8 day course of therapy. Forty to sixty percent of TBSAB should not be treated for two (2) weeks; however, sixty percent of TBSAB requires treatment for thirty days [57].
Supplements
Vitamin C is known to have an endothelial protective effect by preventing tight junction damage and occludin phosphorylation, which in turn reduces capillary hyperpermeability and improves blood flow in the microcirculation [58]. With current knowledge, we would require one daily dose of 3 g/d (30 times the recommended daily dose) for three to six days to restore the levels of vitamin C in the aftermath of the thermal aggression. A study conducted in 2000 by Tanaka et al. [59],demonstrated that early in the resuscitation phase, high doses of vitamin C delivered parenterally (0.66 mg/kg/h per 24 hours) reduced the requirement for fluid, tissue swelling, body weight gain, and days spent on mechanical ventilation. In a 2012 retrospective assessment, Khan et al. [60] demonstrated that large doses of vitamin C administered to 40 critically ill burned patients with more than 20% TBSAB enhanced urine output and decreased the requirement for fluids. In a similar vein, these scientists demonstrated that high vitamin C dosages were safe and would not increase the risk of renal failure.
Higher doses should be taken into consideration in this case, as recommended by Mayes et al. [61], as the standard nutritional therapy appears to be insufficient to correct the levels of 25-hydroxyvitamin D in critically ill burned patients. Nevertheless, few randomised controlled trials (RCTs) have been carried out to date evaluating supplements with high doses of vitamin D administered orally, enterally, or parenterally to critically ill patients. Putzu et al. (2016) [62], conducted two systematic and meta-analytic reviews to evaluate vitamin D supplements in the ICU setting; Weng et al. (2016) [63], conducted a third review (Langlois et al. [64], after adding six RCTs [n = 695]). These reviews found no differences in the rate of death, the number of ICU or hospital stays, or the number of days spent on mechanical ventilation. A tendency towards lower death rates (p = 0.12) and shorter ICU stays (p = 0.12) may be related to the usage of dosages greater than 300,000 IU/d, according to the subgroup analysis [64].
The administration of calcium and vitamin D3 to adult burn victims occurred outside of the crucial phase of the illness. In this regard, Rousseau et al. [65], discovered that osteopenia and hypovitaminosis D were prevalent in these patients and that enhanced quadriceps strength was linked to the administration of large doses of vitamin D upon ICU discharge. The coadministration of vitamin D3 together with a training programme enhanced muscle strength, lean body mass, and serum levels of vitamin D in 48 burned children, according to research published this year by Ebid et. [66].
Synchrotic and probiotic
A condition known as dysbiosis is characterised by overgrown pathogenic bacteria and a concurrent decrease in the microbiome's "health-promoting bacteria," or Lactobacillus and Bifidobacterium [67]. In an effort to restore intestinal balance, probiotic and synbiotic supplementation has been approved as a treatment in a number of critically ill patient populations. Probiotic use decreased the incidence of ICU-acquired infections (RR: 0.80; 95% CI: 0.68– 0.95, p=0.009) and pneumonia related to mechanical ventilation (RR: 0.74; 95% CI: 0.61–0.90, p=0.002), according to a recent study by Manzanares et al. [68], after including 30 RCTs in adult critically ill non-burned patients. Mayes et al. (2015) [69], gave 20 critically burned children a daily dose of 2×105UFC/ ml of Lactobacillus gorbach goldin against a placebo within 10 days after their ICU admission due to the lack of research on probiotic therapy in burned patients. In terms of fewer infections, antibiotic use, or digestive tolerance, the study's findings did not demonstrate any clinical benefits [69].
Due to all of these factors, research on probiotics that involves extremely sick burn victims does not actually provide solid proof. In a similar vein, the actual recommendations include no information regarding the type of probiotic to be used or when to begin therapy-the exception being Saccharomyces boulardii [70]. In this regard, it should be noted that patients who have received Saccharomyces boulardii as a probiotic have been found to be at risk for 40% of invasive infections (fungemia and infective endocarditis) caused by Saccharomyces, and that probiotic use has resulted in cases of fungemia in burn patients [71]. Consequently, Saccharomyces boulardii is not regarded as a probiotic in critically ill patients and should not be given to severely burned patients.
In light of this, ecoimmunonutrition with probiotics and synbiotics in burned patients is still not advised, and more carefully planned clinical trials are still required in this group of extremely sick patients.
Hyper-metabolism and hyper-catabolism: pharmacomodulation techniques
In recent decades, a number of pharmacological approaches-which are not nutritional in nature-have been used to decrease catabolism and/or enhance anabolism in critically ill burn patients. [32,34]. The medications investigated in this context included various anabolic hormones, including growth hormone, insulin, oxandrolone, testosterone, insulin-like growth factor-1 and its associations, insulin-like growth factor-binding protein 3, and specific medications like metformin and propranolol. Among the previously listed therapy approaches, beta-adrenergic blocking has been the most researched and is the only one that the American Burn Association (2013) [72], has determined to be the most effective in controlling these patients' metabolic responses to stress. The reason for this is that, during the burn, catecholamine levels rise significantly, up to ten times greater than baseline. This could partially explain the elevated basal metabolic rate. Propranolol, a non-specific beta blocker, can reduce thermogenesis and EE at rest in order to attenuate sympathetic hyperactivity [73]. However, it also reduces peripheral lipolysis and improves protein synthesis effectiveness by encouraging better protein kinetics in skeletal muscle, resulting in an improved net protein balance that increases by nearly 82%. Additionally, Williams et al. [74], demonstrated that after a burn, propranolol at a dose of 4 mg/kg/d lowers cardiac output and cardiac work without changing average blood pressure. Propranolol treatment prevented the development of hepatomegaly, which is caused by a decreased hepatic absorption of free fatty acids, in children who had burns that covered more than 40% of their TBSAB, according to research by Barrow et al. [75]. In a fascinating systematic review of medical literature conducted back in 2014, Núnez-Villaveirán et al. [76], found 15 RCTs that evaluated the effects of propranolol in burn patients, the majority of whom were children and teenagers. According to a study of these clinical trials, the range of doses that were first used to lower the heart rate by 15–20% has shifted, ranging from 4 to 6 mg/kg/d when given orally, enterally, or parenterally.
The results of a fresh systematic review and meta-analysis involving 10 trials, 9 of which were RCTs and 1062 patients were published more recently by Flores et al. [77]. Propranolol use was associated with statistically significant reductions in basal EE (Hedges’ g=−0.64, 95% CI: −0.8 to −0.5; p<0.001), abdominal fat (g=−0.3, 95% IC CI: −0.4 to −0.1; p<0.001), and peripheral lean mass (g=−0.45; 95% CI: −0.3 to −0.6; p<0.001). The propranolol medication, which was initiated 48 hours to 12 days after thermal damage and was intermittently continued between 10 days and 12 months (averaging 21 days), was found by the authors to be a safe course of action [77].
Shorter hospital stays, gains in body weight, strength and muscle mass, injury healing, and bone metabolism have all demonstrated the benefits of using oxandrolone (0.1 mg/kg every 12 hours) over varying time periods ranging from 6 to 12 months [78]. These effects have been especially noted in burn patients during the acute and rehabilitation stages of their conditions [79].
Currently, 1100 patients are planned in the University of Texas Medical Branch study Assessment of the Treatment of Severely Burned with Anabolic Agents on Clinical Outcomes, Recovery, and Rehabilitation (NCT00675714) [80], which aims to evaluate the early use of ketoconazol, oxandrolone, propranolol, and recombinant growth hormone as a single or combined strategy.
Operative and postoperatively thermoregulation: Thermoregulation during burn debridement or grafting has become one of the key factors affecting metabolic responses. Evaluating the postoperative metabolic aftermath involves changes in thermoregulation as well. The impact of anaesthetic medications, opening bodily cavities, and loss of the majority of the normal regulated systems of control all affect thermoregulation during surgery [8].
The various patterns of postoperative metabolic response can be partially attributed to anatomical and physiological variations in young ones, and adulthood thermoregulation [6].
To restore equilibrium metabolism in a short period with the least amount of loss, it is advised to have expertise in the way the body responds to trauma and procedures in cases of severe stress like trauma and sepsis, recognise modifying metabolic specifications, comprehend the importance of alterations in metabolism for existence during serious illnesses, and aim to reduce catabolic retaliation Practitioners should educate themselves on potential post-traumatic metabolic effects of medications.
Acute malnutrition with severe hypermetabolism and hypercatabolism is modelled in patients with serious burns. Better prognoses for these patients have been attained through accurate demand assessment and matched energy and protein supply based on illness development. It is recommended that burn patients receive large doses of vitamin C and antioxidant cocktail supplements via the parenteral route during the resuscitation phase. These should be given sporadically and consistently based on the TBSAB. These recommendations are based on current knowledge and indicate that early enteral feeding is the best course of action. Further strong evidence is still needed to support enteral glutamine delivery, even if it appears to be a safe tactic capable of optimising therapeutic treatment. Lastly, there have been demonstrated clinical improvements in the group of badly burned patients who have used modified non-nutritional techniques of hypermetabolism and hypercatabolism, specifically propranolol and oxandrolone.
Antagonism between interests
There are no conflicts of interest that the authors of this work have disclosed.
Acknowledgment
Not applicable
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at,Google Scholar,Crossref
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, CrossRef
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Citation: Hassan A. Saad. Nutrition Therapy and Metabolism in Severe Burn. J Diabetes Metab, 2024, 15(3): 1098.
Received: 22-Feb-2024, Manuscript No. jdm-24-29720; Editor assigned: 24-Feb-2024, Pre QC No. jdm-24-29720(PQ); Reviewed: 08-Mar-2024, QC No. jdm-24-29720; Revised: 12-Mar-2024, Manuscript No. jdm-24-29720(R); Published: 19-Mar-2024
Copyright: © 2024 Saad HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.