Research Article - (2016) Volume 4, Issue 2
The present study aimed to elaborate matrix tablets from powder mixture (2:1 ratio) of Algerian date (Phoenix dactylifera L.) fruit and lyophilized berries (Arbutus unedo L.) (LB), using the direct compression technique. In a first part, the physicochemical properties, including the X-ray diffraction, of individual powders and their mixture were determined. In the second step, the swelling, erosion and in vitro release rate characteristics of tablets were studied. The dissolution study of tablets is evaluated throughout the electric conductivity (EC) of surrounding medium (distilled water). Among the four tested models, namely zero-order, first-order, Higuchi and Korsmeyer-Peppas, the latter seems to be the most appropriate (R2=0.972-0.989) to describe kinetics of the ionic transfer whatever the applied temperature. Further, the activation energy (17.272 kJ/mol) related to the transfer mechanism is obtained from the Arrhenius plot with a correlation coefficient greater than 0.899. Globally, the physicochemical parameters of obtained tablets were found to be in compliance with the pharmacopoeial standards.
Keywords: Arbutus berries; Date fruit; Tablet; Electric conductivity; Dissolution; Model
The use of wild plants as food source is well known in all countries of the world, particularly during the periods of war and /or drought. Currently, the research results highlight the significance of the wild edible plants as cheap source of nutrients [1,2] which can explain the interest for using these species as dietary supplements [3]. However, many in vivo studies have evaluated the beneficial effect of the plants on human health.
Wild edible fruits were found to have significant amounts of bioactive compounds, including polyphenols and flavonoids [4]. The high quantities of anthocyanins and natural pigments make them potential candidates for functional food statute [5]. In this context, some wild fruits in India have been identified to have better nutritional value than cultivated fruits [6]. The richness of the wild fruit in valuable ingredients as Iron, Sodium, Potassium, Zinc and Calcium indicate the scope of using wild edible fruits for dietary supplement [7,8].
The Mediterranean region is rich of locally grown, wild and semiwild edible fruit plants. The strawberry tree (Arbutus unedo L.) is one of the typical Mediterranean wild trees, growing in mountains, heavy clay and dry soils, on siliceous and decarbonated substrata [9]. In fresh form, its berry fruits are always incorporated into yogurts and used as confectionaries for pie, pastry fillings and cereal products [10]. After processing, they are also employed for the production of alcoholic beverages, jams, jellies and marmalades [10,11]. Like other plants which are fitted with wonderful defense system assured by various biopharmaceuticals [12], the berries are also known to be used in folk medicine as antiseptic, diuretic and laxative and against cardiovascular pathologies [9]. Wild fruit of Arbutus unedo L. is rich in numerous nutriments specially Calcium, Phosphorus and Potassium [13]. Its sugar content is about 0.47 g/g dry basis (db) of which sacchararose (87.7 ± 0.6 g per kg of dry fruit) and fructose (208 ± 2 g per kg of dry fruit) are the major carbohydrates in the unripe and ripe stage, respectively [14]. Strawberry tree fruits are a good source of antioxidants [15], including carotenoids, flavonoids, anthocyanin and ellagic acid. Then proanthocyanidins accounting for more than 80% of the total flavonoid in arbutus [14-16]. On the other hand, Rodríguez et al. [17] have earlier reported that the higher antioxidant potential of the arbutus berries may be due to the activity of various bioactive components, including vitamin C. In this context, the reducing power of the strawberry tree fruits was found to be one of the highest, among 27 Algerian fruits [18]. So, considering the dietary ingredient, any herbal or botanical material containing vitamins and minerals [19], arbutus berries may be listed as a dietary supplement.
In the last decade, many food products are processed and commercialized in powder form, whereas scientific investigations of various foods powder properties remain insufficient despite their importance in the engineering field [20]. In addition, studies about tableting properties of whole fruits are very scarce: date (Phoenix dactylifera L.) [21], guava and pitaya [22], chebula [23], baobab [24] and mango [25].
The main objective of the present study concerns the tableting ability of the powder mixture (2:1 ratio) of Algerian date (Phoenix dactylifera L.) fruit and lyophilized berries (Arbutus unedo L.) (LB), using the direct compression technique. Some preliminary results have been already communicated and published [26,27].
Fruits and fruit powders
Fully ripe berries were randomly picked at various trees in Kabylie region (North Algeria) during the winter 2013. The fruit was submitted to freeze drying at -64°C under vacuum (4.5 Pa) during 48 h, using lyophilizer Type (Christ Alpha1-4LD), provided with vacuum pump (RZ 6, max pressure 0.04 Pa). The dried product is ground, sieved (sieve of type Euromatest-Sintoo, NFX11-501) to obtain powder with particle diameters (200 ≤ Ø ≤ 400) μm and then kept in closed glass flask at 4°C.
Mech-Degla date fruits were purchased from Boumerdès city (50 km east Algiers). The dates were first cleaned, pitted and cut in small size pieces that were dried at (40 ± 1)°C in laboratory oven (type MELAG 405) until a constant weight was reached. The date powder (DP) (200 ≤ Ø ≤ 400) μm is kept into a hermitic glass at 4°C.
The particle size distribution, Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) of LB, DP and DP/LB mixture were investigated using Malvern Mastersizer 2000 laser diffraction analyzer, FTIR Spectra 2000 (Perkin Elmer) and diffractometer (Panalytical Xpert Pro®.) respectively.
Physicochemical properties of powders
The three powder types DP, LB and DP/LB (2:1 ratio) mixture were characterized first for their bulk (ρbulk) and tapped (ρtab) densities, according to the European Pharmacopoeia [28] by means of an apparatus of type ERWEKA (Engelsmann, Germany).
The Carr index (CI) [29] and the Hausner (HR) ratio [30] were calculated using the following equations:
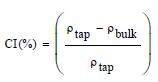 (1)
(1)
 (2)
(2)
The flow rate (g/s) was determined by measuring the time (t) of passage of 100 g of powder through a standardized glass funnel as described by the European Pharmacopoeia [28]:
Flow rate (g/s)=W/t (3)
At the same time, the angle of repose (θ) formed by the cone of the powder onto the flat surface at the exit of funnel was expressed by arctan (θ).
Tablet preparation and basic physical-chemical characterization
Tablets from DP and LB mixture (2:1 ratio) were obtained by compacting the powder using semi alternative tableting press (Mark ED Frogerai. type OA 307).
Tablets processing and their physical characterization were performed in laboratory of CRD/SAIDAL (Algiers) according to the European Pharmacopoeia [28].
• The hardness of 10 tablets was determined with an automatic hardness tester type Pharma Test.
• The friability of the tablets was evaluated with a Friabilator of type ERWEKA TA. For weight uniformity test, 20 tablets were randomly selected, weighed and weight variation (%) was calculated.
• The disintegration time was evaluated on 6 tablets using a disintegrator ERWEKA control ZT 2. The disintegration is considered achieved when 6 tablets are completely disintegrated.
• The swelling ability was quantified through liquid uptake by the tablets which were placed in 3 different liquid mediums (distilled water, 0.1 N HCl and phosphate buffer pH 6.8) heated at 37°C.
• The erosion test is immediately performed after the swelling and consists of the determination of the dried weight of wet tablet by drying at 50°C during 24 h according to Adiba et al. and Zea et al. [21,22]. The morphological examination of tablets during their immersion in distilled water was carried out using a digital camera (Sony®DCRSX65E).
Modeling of dissolution kinetics
Dissolution study was performed according to the method described by Yao et al. [31] with some modifications. Glass beakers (500 mL capacity) containing dissolution medium (distilled water, 0.1 N HCl and phosphate buffer pH 6.8) were placed in a thermostatic magnetic water-bath and stirred at 75 rpm. The kinetic of ions release (at 27, 37, 47 and 57°C) was investigated by studying the variation of the electric conductivity (EC) of the simulating physiologic media, using CONSORT C863 conduct meter. Only results related to distilled water are presented here. The conductivity measurement to follow the dissolution of tablets was developed by many authors [31-36]. On the other hand, the dissolution phenomenon has been already investigated on the tablets from lyophilized berries, using however other models (Peleg, Singh et al. and Singh and Kulshrestha) (submitted, in second round review).
The electrical conductivity of the solution is directly related to the concentration of ions of solids dissolved in the water. However the total dissolved solids (TDS) is a measure of the combined content of all inorganic and organic substances contained in a liquid solution. The ability of the electrolyte to diffuse from the matrix to the surrounding medium can be measured using a conventional conductimeter or TDS meter. The relationship of TDS and specific conductance can be approximated by following equation:
TDS=keEC (4)
The four following models were tested to describe the experimental data
Zero-order model [37]: TDS=TDS0+k0t (5)
Higuchi model [38]: TDS=TDS0+k0t1/2 (6)
First-order model [39]: lnTDS=lnTDS0+k1t (7)
Korsmeyerñ Peppas Model [40]: TDS/TDS0=ktn (8)
where ke=0.53 represents the conversion factor which varies between 0.5 and 0.8 [41], EC (μS/cm), TDS0and TDS are the initial and at any time (t) total dissolved solids (mg/L), respectively; TDS/TDS0 is the fraction of the total released solids at time t; k0, k1, kH and k are the kinetics constants that measure the release rate for zero-order, first-order, Higuchi and Korsmeyer-Peppas model, respectively, n is a diffusional exponent that depends on the release mechanism and the geometry of the system. The value of n for cylindrical shaped matrices is given in Table 1 [42].
| Diffusion exposent (n) | Overall solute diffusion mechanism |
|---|---|
| 0.45 | Fickian diffusion |
| 0.45 < n < 0.89 | Anomalous (non-Fickian) diffusion |
| 0.89 | Case-II transport |
| n ˃ 0.89 | Super case-II transport |
Table 1: Diffusion exponent and solute release mechanism for cylindrical shape.
It must be noticed that these models are commonly used to determine the drug release/dissolution profile from solid dosage forms [43].
Finally, the temperature dependence of the constant (k) corresponding for each model was determined from the Arrhenius equation:
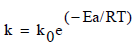 (9)
(9)
Where ko is a pre-exponential factor, Ea the activation energy (kJ/mol), R the universal gas constant (8.314 J/mol K) and T the thermodynamic temperature (K). The activation energy Ea and the constant k0 are determined from the slope and intercept of the plot lnk versus 1/T respectively.
Statistical analysis
All measurements were performed in triplicate. The statistical analysis of the experimental data was performed using Origin software version 8. The Goodness of fit of the selected models was evaluated by the correlation coefficient (R2), the chi-squared error (χ2) and the root mean square error (RMSE):
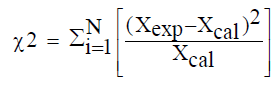 (10)
(10)
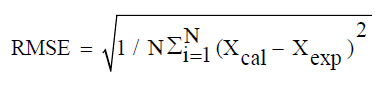 (11)
(11)
Where Xexp is the experimental value, Xcal the value predicted by the model and N the number of experimental measurements.
Powder properties
The physical properties of DP, LB and their mixture DP/LB (2:1 ratio) from which tableting is carried out, are summarized in Table 2. The results show that the LB, DP and their mixture possess excellent flowness ability. However, all powders present CI, HR and angle of repose values below the required limits i.e., 15%, 1.25 and 40°, respectively [28,44] which facilitate the die cavity filling and tableting process [45]. At the same time, the HR values of DP, LB and DP/LB are lower than those of maize (1.57) and wheat (1.81) starch which were experienced as pharmaceutical excipients in tablet formulation [29], but the found values were comparable to that of Azadirachta indica fruit powder (~ 1.41) reported by Mehrotra et al. [46] who have also found an angle of repose of 39.55°, higher to that determined in the present work.
| Ingredients | |||
|---|---|---|---|
| Parameters | LB | DP | LB/DP |
| Bulk Density (g/cm3) | 0.431 ± 0.002 | 0.535 ± 0.003 | 0.438 ± 0.003 |
| Tapped Density (g/cm3) | 0.481 ± 0.004 | 0.562 ± 0.005 | 0.512 ± 0.003 |
| Carr’s index (%) | 10.395 ± 0.005 | 4.850 ± 0.014 | 14.452 ± 0.005 |
| Hausner’s ratio | 1.116 ± 0.006 | 1.050 ± 0.015 | 1.169 ± 0.007 |
| Angle of repose (°) | 26.400 ± 0.782 | 29.93 ± 0.51 | 24.740 ± 0.182 |
| The flow rate (g/s) | 10.291 ± 0.193 | 9.511 ± 0.053 | 8.960 ± 0.061 |
| d10 (μm) | 62.223 | 183.135 | 147.974 |
| d50 (μm) | 190.610 | 294.382 | 310.649 |
| d90 (μm) | 341.908 | 453.121 | 518.176 |
| dn = (d90 – d10) / d50 | 1.467 | 0.917 | 0.714 |
Date are represented as mean ± SD (n=3).
d10, d90 and d50 is the diameter at 10, 90 and 50 % undersize respectively; dn used as an indicator of distribution width
Table 2: Properties of formulation ingredients LB, DP and mixed DP and LB (2:1) powder.
The size distributions of the three powders present a similar general shape as that shown in Figure 1. Moreover, from the data of Table 2, the different size expressions (d50, d50 and d90) vary widely, according to the type of powder. In all cases, the values related to DP are higher than those of LB and this can be simply explained by the compositional characteristics of the initial fruits; the DP/LB mixture displays the greatest d value, considering the predominance of DP in the mixture.
The FTIR spectroscopy is known to be a method commonly applied for characterizing food powders [47-49]. The spectra of LB, DP and (DP/LB) mixtures (Figure 2) attest that there is no appearance or disappearance of peaks in the powder mixture which confirm the absence of any chemical interaction between DP and LB, thus indicating that these powders are compatible [50]. The FTIR spectra also revealed characteristic signals corresponding to various broad and intense bands; in particular, five specific peaks can be mentioned. The intense band in the 3400-3500 cm-1 region is assigned to stretching (υ) vibrations of hydroxyl (-OH) group (free and intermolecular hydrogen band), whereas that around 2930 cm-1 is attributed to (C-H) absorption and include -CH, -CH2, and -CH3 stretching and bending vibrations. The peaks in the region (1630-1061 cm-1) are due to vibrations of the carboxylate (-COO-) group and stretching vibrations of C-O-C bonds in ethers or related compounds respectively [51]. In addition, the FTIR spectra shows another specific peak at around 600 cm-1 which could be assigned to stretching (υ) of (-CH2) and (-CH) groups as previously suggested by Kamil et al. [52] for tomato products.
The XRD patterns of DP, LB and the mixture of powders (DP/LB) are presented in Figure 3. A very broad band with very weak peaks, characteristic of amorphous forms, is observed in the spectrum of LB (Figure 3a). On the contrary, in the case of DP, the diffraction peaks are intense, indicating the presence of a crystalline structure (Figure 3b). We think that the high sugar content of DP may be the principal responsible of the enhanced crystallinity. The XRD pattern of DP/LB mixture (Figure 3c) exhibited peaks corresponding almost perfectly to those of DP. In opposite, LB picks are not clear, being probably masked by the high DP peaks. On top of that, the effects of compression and sample high hygroscopicity probably alter the XRD analysis as reported by Ledur Alles et al. [53] on saccharides powder from yak on roots (Smallanthus sonchifolius). Furthermore, the amorphous characteristics are clearly reported on different dried mango powders [54] and fluidize-dried gum extracted from the fresh fruits of Abelmoschus esculentus [55]. However, Niimura et al. [56] have shown that strawberry flesh has low-crystallinity cellulose I. According to these results, it can be concluded that the crystallinity depends on both the nature of the extract and composition of fruits.
Some physical-chemical characteristics of tablets
The physical characteristics of prepared tablets are presented in Table 3. Crushing strength test demonstrates the ability of tablets to withstand pressure or stress during handling, packaging and transport. Furthermore, the mechanical strength of tablets determines the disintegration time and the dissolution rate.
| Properties | Value |
|---|---|
| Hardness (kp) Friability (%) Weight (g) Time disintegration (min) Diameter (cm) Thickness Humidity (%) |
9.670 ± 0.810 0.102 ± 0.003 0.502 ± 0.002 25.00 ± 3 1.260 ± 0.010 2.000 ± 0.005 1.75 ± 0.020 |
Date are represented as mean ± SD
Table 3: Technological Characterization attributes of the prepared tablet (mixture DP/LB).
The hardness and friability of obtained DP/LB tablets met the minimum requirement to be within the Pharmacopoeial limits (˃ 4 kp and ˂ 1% respectively), thus confirming the ability of LB/DP powder mixture (2:1 ratio) for: i) tableting application without adding any chemical blinder, and ii) withstanding the mechanical shocks during their handling and transport.
The disintegration time (25 min) of the matrix is comparable to that (24 min) found by Niimura et al. [56] about the disintegration of tablets from noni (Morinda Citrifolia, L.) fruit extract added with maltodextrin as sub coating material, and it is less than (˃ 30 min) that found by Ngwuluka et al. [57] regarding the disintegration of paracetamol tablets added with the dried fruit of date (Phoenix dactylifera L.) as an excipient.
Dissolution properties of obtained tablets
Tablet swelling and erosion studies: Tablet swelling and erosion is a valuable test to better understand the mechanisms of release and the relative importance of participating parameters [58,59].
Matrix tablet erosion and swelling kinetics (Figures 4 and 5) demonstrated a linear increase of both parameters up to 20 min, moreover the Zero-Order equation perfectly fit the experimental data (R2=0.99). According to the immersion liquid, the following order of evolution can be establish for erosion and swelling intensities over the dissolution process: distilled water ˃ phosphate buffer ˃ HCl and phosphate buffer ˃ HCl ˃ distilled water respectively. The tablets showed a maximum degree of swelling at 24 min whatever the surrounding media. This swelling phenomenon is governed by the osmotic effect whose mechanism is correlated to that already described by Costa et al. [60]; the water migration takes place from less concentrated to the more concentrated solution. Khan et al. [61] suggested that the swelling kinetics of the matrices were an important determinant of drug release. At the same time, the tablets had the highest erosion rate in phosphate buffer which is in correlation with the swelling results. In all media, the dissolution is a balance between swelling and erosion which determines the release process of ions from the fruit tablets. These findings are in agreement with works of Adiba et al. [21] and Zea et al. [22] concerning food tablets prepared with date (Phoenix dactylifera L.)/spirulina (Spirulina sp.) and guava/pitaya powders respectively.
Dissolution and kinetics modeling: Electrolyte content increases with dissolution time and temperature. All the curves present the same evolution of dissolution process with two distinct periods: the first phase of 15 min. corresponds to the increase in the cumulated conductivity while the second equilibrium phase indicates a suspension of any transfer of matter (in terms of electrolytes). This last phase corresponds in fact to the complete dissolution of the tablet. Buckton and Efentakis [61,62] and Ferrero et al. [63] have obtained similar curves during the use of thermodynamic activation parameters to describe and characterize the mechanisms of released drug from hydrophobic matrices.
At 57°C, the EC change is higher, compared with other temperatures. However, at the equilibrium phase EC is found to be 3.5 and 2.5 times higher than those at 27 and 37°C respectively. The effect of temperature on the conductivity agrees with the published studies [64] where the authors demonstrate that the leaching of inorganic dissolved solids in the solution increases with increasing temperature.
The modeling results of the release kinetics of TDS in the case of distilled water as dissolution media are recapitulated. Incontestably, for all temperatures: 27, 37, 47, and 57°C, Korsmeyer-Peppas model fit better the experimental data with (R2=0.989 with RMSE<0.1 and χ2<0.05). Adiba et al. [21] and Ong et al. [25] have used only the Korsmeyer-Peppas model and found a good fit. But, working on tablets from 80% pectin, Khule et al. [65] have found that among the four tested models, that of Korsmeyer-Peppas was the best. On the other hand, Baddam and Bandela [66] suggested that the Korsmeyer-Peppas model is suitable for predicting the dissolution times of several tablets.
The 0.447 value of the exponent (n) for Korsmeyer-Pappas model at 27°C indicates that the mechanism of released ions from the tablets is of a Fickian type, controlled by diffusion mechanism. At higher temperatures, the calculated exponents (n) were in the range from 0.601 to 0.606 (better for each temperature), indicating either an anomalous transport or non-Fickian mechanism. These results are in concordance with those of Cruz et al. [67] about polyethylene oxide/ primaquine matrix tablets which showed that the ions being released by both diffusion and erosion are controlled mechanism.
Effect of temperature: To the best of our knowledge, there are no reports available on the investigation of the temperature dependence of ionic transfer including food matrices. The attempts to model drug release profiles through the thermodynamic parameters are limited. However, Buckton and Francis [68] confirmed that is possible to study and describe the dissolution mechanism of drug released from a controlled oral dosage form, by use of the thermodynamic parameters of activation. It can observe that the zero order models leads to a maximal activation energy followed by both the Higuchi and first model. The low value is obtained in the case of the Peppas model (17.272 kJ/mol). This value is very close to that (18.3 kJ/mol) related for the diffusion drug process (sodium salicylate) from non-disintegrating hydrophilic matrices Touitou and Donbrow [69] and those reported by Ferrero et al. [63] in the case of caffeine released from hydrophilic matrices made of cellulose ethers. These authors have shown that the apparent Ea values (14.4 ± 4.0 and 16.3 ± 2.5 kJ/mol for pH 6 and pH 2) respectively. They have concluded that these values are low and not compatible with a swelling-controlled process mechanism which can be assigned to the dimensional change of the system during drug release.
Powders from Strawberry tree (Arbutus unedo L.) fruits, date (Phoenix dactylifera L.) fruits and their mixture with 1/2 ratio were first investigated for some of their physical properties. Based on X-ray diffraction analysis, the powder mixture shows a crystalline structure, similar to that observed for date fruit powder. The results also revealed the good ability of the powder mixture for tableting by direct compression. In addition, it was found that among the four applied models, the Kosmeyer-Peppas better describes the experimental data of the dissolution kinetic of the tablets, the dissolution process being evaluated throughout the total dissolved solids via the electric conductivity measurement of the surrounding media. The temperature dependence of the dissolution process is studied from; it was estimated using Arrhenius equation. The latter allowed concretely to determinate the activation energy (17.272 kJ/mol) using the rate constant of the selected model. Taking into account the nutritional and physiological potentials of the basic components of the analyzed powder mixture, the obtained tablets may be used as dietary supplement and/or as excipient in the pharmaceutical industry. Additionally, the physicochemical characterizations (toxic elements, behavior of tablets in various solutions simulating the physiological mediums, photochemical screening) are in the process of finalization and the results will be communicated in a future paper.
This work is supported by the thematic research agency in science and technology (ATRST, ex. ANDRU Algiers / Algeria) as part of an innovative project.