Research Article - (2021) Volume 9, Issue 9
The aim of this work is to isolate, characterize, and evaluate the antioxidant activity as well as the phytotoxicity of crude extracts and compounds obtained from C. febrifuga. Nine compounds were isolated from leaves, stem bark and roots (methylene chloride/methanol:1/1) crude extracts after repeated silica gel column chromatography corresponding to shanzhiside methyl ester (1), D-mannitol (2), a mixture of two isomeric pentacyclic triterpenes: oleanolic acid (3a) and ursolic acid (3b), 11-methylixoside (4), 3β-urs-12, 20(30)-diene-27,28-dioic acid (5), quinovin glycoside C (6), 3-O-β-D-3-oxo-glucopyranosyl-ursa-12,20(30)-diene-27,28-dioic acid (7) and 3β-(-α-Lrhamnopyranosyloxy)-ursa-12,20(30)-diene-27,28-dioic acid (8). The compound chemical structures were established by studying their 1H and 13C NMR (1D and 2D) and Mass Spectrometry (MS) spectra. The antioxidant capacity of extracts were evaluated using a recently developed electrochemical method consisting in interacting with the superoxide anion radical (O2.-) generated in situ by reduction of air molecular oxygen (O2) in aprotic medium (anhydrous DMF). The antioxidant index is expressed as the amount of extract needed to consume 30% (AI30) of electrogenerated O2.-. The lower is the antioxidant index, the more active the substrate is against O2.-. The stem bark crude extract is the most active with an AI30 of 79.50mg/L while the leaves and root crude extracts showed a moderate activity against O2.- with AI30 of 303.70mg/L and 450.00mg/L respectively. The phytotoxicity study were carried out using a germination test on Lepidium sativum seeds in absence (0mg/L) and in presence of increasing concentrations (10, 100, 500, 1000mg/L) of crude extracts and the isolated compound (1). The following physiological parameters of the seedlings were evaluated 72 hours after the experimentation: germination percentage (G %), germination index (GI), vigor index (VI), relative root growth (RRG %). Root extracts are the most phytotoxic on seed germination with G% ranging from 66.66 to 100% and RRG% from 8.20 to 88.45% while the isolated compound (1) stimulated growth between 10-500mg/L but showed a moderate phytotoxic effect on seedlings above 500mg/L.
Crossopteryx febrifuga; Crude extracts; Isolation; Superoxide radical anion; Antioxidant activity; Phytotoxicity.
(AI) Antioxydant Index; (AI30) Antioxydant Index needed to consume 30% of O2 in the process; (Bu4NPF6) Tetrabutylam- monium HexafluoroPhosphate; (°C) Degree Celsius; (13CNMR) Carbon-13 Nuclear Magnetic Resonance; (CC) Column Chro- matography; (CH2Cl2) Ethylene Chloride; (CM) Centimeter; (Conc) Concentration; (COSY) Correlation-Spectrocopy; (CV) Cyclic Voltammetry; (1D) One Dimension; (2D) Two Dimen- sions; (D) Doublet; (dd) Doublet of doublets; (ddd) Doublet of Doublet Deboubled; (DI) Insaturation Degree; (DMF) Dimetth- ylformamide; (DMSO) Dimethylsulfoxide; (DMSO-d6) Dimethyl sulfoxide hexadeutered; (EtOAc) ethyl acetate; (dt) Doublet of triplets; (g) Gram; (G%) Germination percentage; (GI) Germination index; (GPES) General Purpose Electrochemical System; (1HNMR) Proton Nuclear Magnetic Resonance; (H2SO4) Sulfuric Acid; (HMBC) Heteronuclear Multiple Bond Connectivity; (HRESI-MS) High Resolution ElectroSpray Ionization Mass Spectra; (HSQC) Heteronuclear Single Quantum Coherence; (Ip) Peak current; (Ipa) Peak anodic current; (IR) Infra-Red; (J) Coupling constant; (KBr) Potassium bromure; (Kg) kilogram; (L) Liter; (m) Multiplet; (MeOH-d4) Methanol Tetra Deteured; (MG) Milligram; (M/Z) mass/charge; (MHz) Megahertz; (Min) Minute; (ML) Milliliter; (MM) Millimol Per Liter; (MM) Millimeter; (MS) Mass Spectra; (O) Overlap; (PH) Potential Hydrogen; (PPM) Part Per Million; (ROS) Reactive Oxygen species; (RRG%) Relative Root Growth; (S) Second; (S) Singulet; (TLC) Thin Layer Chromatography; (TMS) Tetramethylsilane; (UV) Ultraviolet; (V) volt; (V/V) Volume/Volume; (VI) Vigor Index; (δ) Chemical Shift; (μL) Microliter; (μS) Micro Siemens.
Crossopteryx febrifuga, etymologically fighting fever, is a mono- specific genus of Rubiaceae family and belonging to the Ixoroideae subfamily [1]. The Rubiaceae have about 630 genera and more than 13,000 species divided into 3 subfamilies including Rubiodeae, Cinchinoideae and Ixoroideae, making this family one of the six largest families of Angiosperms [2]. C. febrifuga is a characteristic looking shrub with opposing branches that can reach 5 to 6 m in height. It gives oval and opposite leaves often reddish at their base, very fragrant white flowers grouped in clusters at the top of the twigs, its round and green fruits turn blackish when ripe. The flowering period is from May to July. This shrub grows in dry forests on the sandy soils of southern Africa [3]. It is known under vernacular names of Ndeubeuh mann by indigenous peoples in southern Chad and Balembo by Bambara peoples in Mali [4]. Balembo is also the name of local antitussive syrup made with C. febrifuga roasted fruits. This plant is used in African folk medicine to treat various diseases such as intestinal worms, cough, gout, gonorrhea, fever, diabetes and diarrheal diseases [4-7]. In southern Chad, this species is known to have many therapeutic properties, thus a decoction of root powders is taken against stomachaches. In rural areas, the same decoction is recommended to women after childbirth for its antibiotic effects. The scraping of stem bark, brown in color, is sold in local markets in the form of herbal tea, commonly called Goub, for its taste and medicinal properties while a decoction of young leaves is taken as antipyretic against fever attacks. The leaves are also used in traditional veterinary medicine as a dewormer for livestock. This species is actually threatened with extinction in Chad due to its excessive use in folk medicine and overgrazing. Chadian Government recently raised the possibility of creating a botanical garden in collaboration with the National Association of Traditional Healers in order to safeguard interesting wild plant threatened with extinction. This measure could save C. febrifuga in southern Chad. Some recent phytochemical studies revealed the presence of iridoids, phenols, flavonoids, tannins, triterpenes, saponins and steroids in C. febrifuga extracts [8-10]. Secondary metabolites with high pharmacological potentials isolated from Rubiaceae justify the growing interest in the scientific investigation of the species in this family [11]. Despite the plant's many therapeutic prop- erties recognized in ethnopharmacology, relatively few studies have been carried out in order to validate them scientifically. In this study, we evaluated for the first time the phytotoxicity of the crude extracts and isolated compound (1) on the germination and growth of Lepidium sativum seeds. The antioxidant capacity was measured according to a relatively new electrochemical method consisting in evaluating the capacity of the substrate to trap the superoxide anion radical (O2●−) generated in situ by reduction of molecular oxygen (O2) [12]. The superoxide anion radical is one of the reactive oxygen species called "ROS" whose unregulated accumulation in the human body can cause various disorders such as cancers, neurodegenerative diseases [13,14]. We also report direct isolation of a saponin (3β-(-α-L-rhamnopyranosyl)-ursa- 12,20(30)-diene-27,28-dioic acid) (8) previously obtained from C. febrifuga extracts by acid hydrolysis of a bisdesmosidic saponin as well as eight other known compounds.
General experimental procedures
The masses were measured on a 1/1000 Sartorius type electronic balance. Column chromatography (CC) was carried out on a high purity grade silica gel adsorbent, pore size 60 Å, 70 mesh-230 mesh, 63-200μm. The extracts were concentrated on a Büchi R-110 brand rotavapor. Thin Layer Chromatography (TLC) was carried out on square aluminum plates (20×20 cm2) covered with silica (0.2 mm thick) of the SIL G/UV254 Merck type, the spots were deposited with capillaries (Blaubrand ® intraMark) of 5 to 20 μL and visualized under a UV lamp at 254 or 236 nm or revealed by spraying a H2SO4 (10%) solution followed by heating in an oven. NMR spectra were recorded on a Brüker Avance III 400 spectrometer operating at 400.13MHz for 1H, equipped with a BBFO probe with a z-gradient coil and a GREAT 1/10 gradient unit. All experiments (1HNMR, 13CNMR and 2D as COSY, HSQC and HMBC) were carried out at 25°C. 1H chemical shifts (δ) are given in ppm relative to the solvent residual peak and 13C chemical shifts are relative to the central peak of the solvent signal. The products to be analyzed were solubilized in deuterated solvents (MeOH-d4 or DMSO-d6). The chemical shifts δ (ppm) were recorded with reference to tetramethylsilane (TMS, δ=0). The MestReNova software was used to process the NMR spectra. The Mass Spectra (MS) are recorded on an Orbitrap Q- Extractive type apparatus (Thermo Fisher Scientific, Waltham (MA), USA) in direct infusion, in Electrospray ionization (+)/(-) modes. A JASCO 320-A type IR spectrophotometer using KBr pellets recorded the IR spectra. The cyclovoltammogram was performed on a Potentiostat Galvanostat (Autolab) Echochemie type measuring device coupled to a computer. The data were recorded and processed by GPES Manager Software (General purpose electrochemical system, version 4.9, Echo chemie B.V). A thermostatically controlled cell containing 10 mL of 0.1 M solution (Bu4NPF6), maintained at 21 ± 0.3°C by a Fisher scientific polystat 5L/8662H type thermoregulator, in which were bathed 3 electrodes playing specific roles, namely a working electrode at glassy carbon disc (polished using a struers Dap-7 polisher fitted with a green Sci-paper, grit 1200, size: 200 mm dia, and rinsed with distilled water then dried after each use), an electrode reference (Ag/ AgCl) in ethanol saturated with LiCl and a counter electrode, in the platinum wire which completes the circuit by leading the electrons through the solution to the working electrode. The germination bioassay was carried out in sterile Pétri boxes (ø 90 mm) containing a layer of filter paper.
Plant material
Leaves, stem bark and root scraping of C. febrifuga were freshly harvested, in March 2014, in Béti, Department of Pendé/Logone Oriental Region, Republic of Chad, after identification by Mr Dodorom Téblé Wolwaï, a botanist at the Faculty of Science and Technics, University of Doba (Chad) and confirmed by the Yaoundé National Herbarium Voucher specimen number 41791/ HNC. These samples were transported to the laboratory and gen- tly cleaned with distilled water to remove dust and then dried for two weeks at room temperature out of direct sunlight.
Extracts preparation
After preliminary stages of drying and manual powdering the plant material; 1.0 kg of powder from the leaves; 1.0 kg of powder from the stem bark and 1.0 kg of powder from the root were separately extracted by maceration, at room temperature, for 48 hours with 6 L of methylene chloride/methanol (1/1, v/v) sys- tem. Maceration is a liquid-solid extraction method that takes place at room temperature and has the advantage of preserving heat sensitive molecules before any intentional modification of their structures in organic synthesis. This operation was repeated twice in order to optimize the extraction. The different resulting solutions were filtered using Whatman paper and then concentrated under reduced pressure at 55°C on a rotavapor to give successively 80 g of a soft green brown paste for the leaves, 80 g of an amorphous solid of chocolate color for the root and 100 g of raw extract of a creamy consistency and red brown in color for the stem bark. These crude extracts were stored at 4°C until the next stage of the study.
Column chromatography and isolation
Samples of 50 g of crude extracts were subjected separately to liquid silica gel column chromatography (pore size 60Å, 70 mesh-230 mesh, 63-200μm, 300 g) and eluted with n-hexane/ ethylacetate and ethylacetate/methanol systems in increasing polarity (100:0→0:100). Fractions of 200 mL each collected at the outlet of the chromatographic column were concentrated using a rotavapor. This operation yielded a total of 218 fractions for the stem bark crude extracts, 340 fractions for the leaves crude extracts and 400 fractions for the root crude extracts and then gathered into major sub-fractions according to their thin layer chromatographic profiles. Successive purifications of some UV absorbing sub-fractions afford nine compounds identified using spectroscopic NMR, Mass Spectrometry methods and corre- sponding to the following known compounds:
Leaves crude extracts
Four compounds were isolated from this extract: Shanzhiside methyl ester (1), a white powder (250.20 mg) precipitated in the EtOAc/CH2Cl2 1:1 system; D-mannitol (2), white powder (19.7 mg, EtOAc/CH2Cl2 1:1); a mixture of oleanolic acid (3a) and ursolic acid (3b); white powder (31.71 mg, n-C6H14/EtOAc 7.5:2.5).
Root crude extracts
Three compounds including 11-methylixoside (4), yellowish amorphous powder (7.52 mg, EtOAc/MeOH 9:1); 3β-urs-12,20(30)- diene-27,28-dioic acid (5), white powder (5.23 mg, n-C6H14/EtO-Ac 3:2) and quinovinglycoside C (6), white amorphous powder (8.63 mg, n-C6H14/EtOAc 1:9).
Stem bark crude extracts
Two compounds including 3-O-β-D-3'-oxo-glucopyranosyl-ursa- 12,20(30)-diene-27, 28-dioic acid (7), brownish powder (11.80 mg, n-C6H14/EtOAc 1:1) and 3β-(α-L-rhamno- pyranosyl)-ursa- 12,20(30)-diene-27,28-dioic acid (8), beige powder (18.0 mg, EtO- Ac/MeOH 9:1) were isolated.
Phytotoxicity test
We evaluated the phytotoxicity of crude extracts and the isolated compound (1) on the germination and growth of Lepidium sativum seeds, a vegetable plant of Brassicaceae family. The study was performed according to the protocol described by Mihaela with a slight modification [15]. For each sample, series of concentrations of 10, 100, 500 and 1000 mg/L were prepared using distilled water. The solutions obtained were further homogenized in an ultrasonic bath. Negative control was made with distilled water containing no substrate (0 mg/L). Ten (10) seeds of regular contours and similar sizes, selected after a visual examination, are inoculated with 5ml of each solution previously prepared in sterile Petri dishes where filter papers were previously placed, serving as the germination surface. The seeds were distributed over the entire surface of the box to prevent any hindrance during the germination period. This operation was repeated 3 times for each concentration. Before the experimentation, physico chemical parameters of solutions such as pH and conductivity (μS/cm) were measured. After 3 days of incubation at room temperature and protected from light, the following physiological parameters of the seedling were measured: germination percentage (G%), germination index (GI), relative root growth (RRG%) and vigor index (VI) expressed as follows [16,17].

In the germination process, we considered as germinated seeds with root lengths ≥ 1 mm. Comparisons with the control (0 mg/L) allowed us to know the allelopathic response of the seedling to the external stress. Allelopathy is any direct or indirect effect, positive or negative, of a plant on another through the production of chemical compounds released into the environment [18]. The allelopathic activity of Brassicaceae family is attributed to glucosinolates, a group of sulfur containing carbohydrate compounds common in this family [19,20].
Determination of the antioxidant activity of the crude extracts
0.1 M solutions (Bu4NPF6) were prepared by dissolving 0.387 g of Bu4NPF6 (support electrolyte to conduct the current) in 10 mL of anhydrous DMF (99.8%) and saturated with dry air for 10 min. Under the operating conditions, the solubility of oxygen in the air is approximately S(O2)=0.94.10-3M. First, the molecular oxygen (O2) reduction in cyclic voltammogram was recorded, in the absence of any antioxidant substrate, at a scan rate of 0.1V.s-1. The initial potential was set at 0 V vs Ag/AgCl. The sweep potential was reversed at -0.9 V relative to Ag/AgCl. The presence of the radical (O2â?− in the medium is easily detected by measuring its anodic oxidation current (Ipa°) during reverse scanning [12]. We then prepared 5 mL of stock solutions (10 g.L-1) each by dissolving crude extracts (root, stem bark, leaves) in anhydrous DMF. Gradual volumes of these freshly prepared stock solutions were added to the 10 mL of 0.1 M solution (Bu4NPF6) in the thermostatically controlled cell to obtain a range of antioxidant substrate concentrations from 0 mg.L-1-700 mg.L-1. After each addition, the cyclic voltammogram of the progressive reduction of the superoxide radical was recorded. The consumption of O2â?− is correlated with the concentration of crude extracts gradually added in the electrochemical cell, thus reflecting their antioxidant capacity. This antioxidant capacity is estimated by the Antioxidant Index (AI30) defined as the concentration of extract required to consume 30% of electrogenated superoxide anion radical. The smaller the AI30, the more active the extract is against the super-oxide anion radical [21].
Structures of the isolated compounds
After a column chromatographic fractionation of the crude extracts followed by successive purifications on sephadex LH20, the following nine known compounds, belonging to four organic compounds groups (iridoids, triterpenes, saponins and carbo- hydrates) were isolated. Their structures were identified by 1D (1H, Mitragyna Stipulosa [29], 3-O-β-D-3'-oxoglucopyranosylC, DEPT135), 2D (COSY, HSQC, HMBC) NMR spectra and by comparison with the literature data: Shanzhiside methyl es- ter (1) [22], D-mannitol (2), a primary metabolite common to several species [23], a mixture of two isomeric pentacyclic trit- erpenes: Oleanolic acid (3a) and ursolic acid (3b) [24-26], 11-me- thylixoside (4) [27], 3β-urs-12,20-diene-27,28-dioic acid (5) [28], quinovin glycoside C (6), an antivenom saponoside previously isolated from extracts of Mitragyna Stipulosa [29], 3-O-β-D-3'-oxo-glucopyranosyl-ursa-12,20(30)-diene-27,28-dioic acid (7) [8] and 3-β-(-α-L-rhamnopyra- nosyloxy)-ursa-12,20(30)-diene-27,28-dioïc acid (8) isolated for the first time from extracts of C. febrifuga (Figure 1).
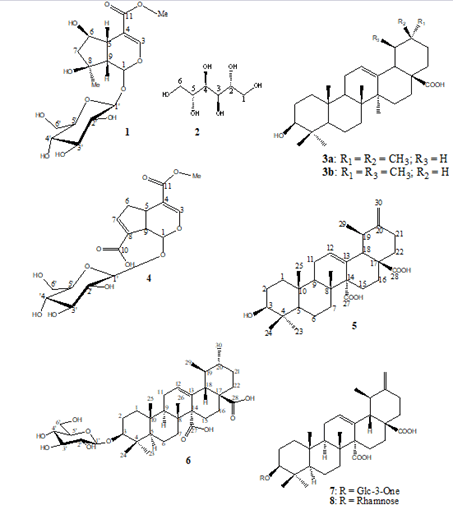
Figure 1: Chemical structures of the isolated compounds.
General informations on the isolated compounds
Compound 1 (Shanzhiside méthyl ester) white powder (ethyl ac- etate/methanol 1:1), (HRESI-MS) (+): m/z 429.13673 [M+Na]+ (calculated for C17H26O11Na: 429.1367) corresponding to the formula C17H26O11, DI=5,1H NMR (δppm, DMSO-d6, 400MHz): 7.33 (d, J=1.25Hz, H-3), 5.50 (d, J=2.3Hz, H-1), 4.45 (d, J=7.9Hz, H-1’), 3.92 (m, H-6), 3.70 (ddd, J=11.9Hz, J=6.4Hz, J=1.9Hz, Ha-6’), 3.65 (s, OMe-12), 3.44 (dt, J=11.9Hz, J=6.0Hz, Hb-6’), 3.15(o) m, H-5’, 3.12 (o) m, H-3’, 3.04 (ddd, J=9.4Hz, J=8.7Hz, 5.3Hz, H-4’), 2.93 (ddd, J=8.7Hz, J=8.0Hz, J=5.4Hz, C-2’), 2.81 (dd, J=9.9Hz, J=2.5Hz, H-5), 2.45 (dd, J=9.8Hz, J=2.3Hz, H-9), 1.83 (dd, J=13.1Hz, J=6.6Hz, Ha-7), 1.63 (dd, J=13.1Hz, J=6.4Hz, Hb-7), 1.11 (s, H-10), 13C NMR (100MHz): 167.19 (C-11), 151.00(C-3), 109.6(C-4), 98.0(C-1’), 92.8(C-1), 77.33(C-5’), 76.99(C-8), 76.7(C-3’), 74.9(C-6), 73.1(C-2’), 70.02(C-4’), 61.09(C-6’), 51.25(OMe-12), 49.99(C-9), 49.18(C-7), 40.29(C-5), 24.7(C-10).
Compound 2 (D-mannitol) white Powder (ethyl acetate/methanol 1:1), 1H NMR (δppm, DMSO-d6, 400MHz): 4.41(d, J=5.6Hz, OH-1/6), 4.34(t, J=5.6Hz, OH-2/5), 4.13 (d, J=7.0Hz, OH-3/4), 3.61(ddd, J=10.7Hz, J=5.6Hz, J=3.4Hz, Ha-1/6), 3.53 (t, J=7.6Hz, H-3/4), 3.45(ddd, J=10.7Hz, J=5.6Hz, J=3.5Hz, H-2/5), 3.39(dd, J=10.7Hz, J=5.7Hz, Hb-1/6), 13C NMR (100MHz): 71.35(C-2/5), 69.71(C-3/4), 63.90(C-1/6).
Compounds 3a (oleanolic acid): White powder (hexane/ethyl ac- etate 7.5:2.5), ESI- m/z 455 [M-H]- compatible with C30H48O3, DI=7, IR (KBr): νmax=3,300cm-1(-OH), 3,000cm-1(Csp3-H), 1,700cm-1(C=O), 13C NMR (125MHz, DMSO-d6): 178.2 (C-28), 143.8 (C-13), 121.5 (C-12), 76.8 (C-3), 55.9 (C-5), 48.2 (C-9), 46.7 (C-17), 46.6 (C-19), 42.2 (C-14), 42.1 (C-18), 39.4 (C-4), 39.8 (C-8), 39.1(C-1), 37.4 (C-10), 34.3 (C-21), 33.4 (C-7/29), 33.2 (C-22), 31.0 (C-20), 28.8 (C-23), 28.4 (C-15), 28.0 (C-2), 26.22 (C-27), 23.8 (C-11/16/30), 18.8 (C-6), 17.6 (C-26), 16.5 (C-24), 15.6 (C-25).
Compounds 3b (ursolic acid): white powder (hexane/ethyl acetate 7.5:2.5), ESI- m/z 455 [M-H]- compatible with C30H48O3, DI=7, IR (KBr): νmax=3,300cm-1(-OH), 3,000cm-1(Csp3-H), 1,700cm-1(C=O), 13C NMR (125MHz, DMSO-d6): 178.6 (C-28), 138.2 (C-13), 124.5 (C-12), 76.8 (C-3), 55.9 (C-5), 53.6 (C-18), 48.2 (C-9), 48.1 (C-17), 42.7 (C-14), 40.1 (C-8), 39.7 (C-4), 39.3 (C-1), 39.5 (C-19), 39.4 (C-20), 37.6 (C-10), 37.4 (C-22), 33.8 (C-7), 31.1 (C-21), 28.9 (C-15), 28.8 (C-23), 28.3 (C-2), 25.0 (C-16), 24.0 (C-27), 23.8 (C-11), 21.4 (C-30), 18.9(C-6), 17.5 (C-26/29), 16.5 (C-24), 15.7 (C-25).
Compound 4 (11-Methylixoside) yellowish amorphous powder (ethylacetate/methanol 9:1), High Resolution ESI Mass spectrum (HRESI-MS) (+)/(-), ESI-: m/z 425 [M+Na]+ and ESI- m/z 401 [M-H]- compatible with the formula C17H22O11, 1H NMR (δppm CH3OH-d4, 400MHz): 7.49(d, J=1.1Hz, H-3), 6.90 (dt, J=2.5Hz, J=1.6Hz, H-7), 5.69(d, J=5.0Hz, H-1), 4.62(d, J=7.9Hz, H-1’), 3.86(m, Ha-6’), 3.71(s, OMe-12), 3.68(m, Hb-6’), 3.36(m, H-3’), 3.33(m, H-5), 3.30(m, H-5’), 3.29(m, H-4’), 3.22(m, H-9), 3.19(dd, J=8.9Hz, 7.9Hz, H-2’), 2.93(dt, J=18.7Hz, 8.0Hz, 2.3Hz, Ha-6) and 2.43(m, Hb-6), 13C NMR (100MHz): 169.2(C-11), 168.0(C-10), 153.5(C-3), 147.5(C-7), 136.2(C-8), 112.4(C-4), 100.4 (C-1’), 96.3(C-1), 78.2(C-5’), 77.8(C-3’), 74.6(C-2’), 71.4(C-4’), 62.6(C-6’), 51.7 (OMe-12), 47.4(C-9), 40.2(C-6) and 35.0(C-5).
Compound 5 (3β-urs-12,20 (30) -diene-27,28-dioic acid) white powder (hexane/ethyl acetate 6:4), (HRESI-MS) (+)/(-), ESI+ m/z [M+Na]+ 507.30809 (calculated for C30H44O5Na: 507.3082) and ESI-, m/z [M-H]- 483.3116 (calculated for C30H43O5) com- partible with the formula C30H44O5(DI=9), 1H NMR (δppm, DMSO-d6, 400MHz): 5.50 (m, H-12), 4.67 (brs, Hb-30), 4.59 (brs, Ha-30), 4.32 (brs, OH-3), 2.95 (brs t, J=7.2Hz, H-3), 2.22 (d,j=11.7Hz, H-18), 2.18 (o, H-9), 2.12 (o, 2H-21), 2.05 (o, Hb-16), 1.98 (o, Ha-15), 1.9 (o, Hb-11), 1.84 (o, H-19), 1.8 (o, Ha-11), 1.70 (o, Hb-15), 1.69 (o, Ha-22), 1.63 (o, Ha-16), 1.6 (o, 2H-1), 1.6 (o, Hb-7), 1.55 (o, Ha-7), 1.46 (o, Hb-22), 1.45 (m, Ha-6), 1.45 (o, 2H-2), 1.25 (o, Hb-6), 0.95 (d, J=6.3Hz, H-29), 0.87 (s, 3H-25), 0.86 (s, 3H-23), 0.79 (s, 3H-26), 0.66 (s, 3H-24), 0.58 (d, J=10.9Hz, H-5), 13C NMR (100MHz): 177.7(C-28), 176.0(C- 27), 152.5(C-20), 132.5(C-13), 128.3(C-12), 105.8(C-30), 77.0(C-3), 55.7(C-18), 55.4(C-14), 55.0(C-5), 47.4(C-17), 46.12(C-9), 39.0(C-8), 38.4(C-1), 38.4(C-4), 38.1(C-22), 36.5(C-10), 36.3(C- 7), 35.0(C-19), 31.5(C-21), 28.34(C-23), 27.0(C-2), 25.15(C-16), 24.1(C-15), 22.5(C-11), 18.2(C-26), 18.0(C-6), 16.1(C-24), 16.0(C- 25), 15.99(C-29).
Compound 6 (quinovin glycoside C): White amorphous powder (hexane/ethyl acetate 1:9), (HRESI-MS)(+)/(-), ESI+ m/z [M+Na]+ 671, ESI- m/z [M-CO2-H]- 603 compatible with the for- mula C36H56O11, DI=9, 1H NMR (δppm, DMSO-d6, 400MHz): 5.49 (m, H-12), 4.14 (d, J=7.8Hz, H-1’), 4.02 (m, H-4’), 3.64 (dd, J=11.0Hz, J=3.4Hz, Ha-6’), 3.41 (o, Hb-6’), 3.1 (t, J=8.6Hz, H-3’), 3.06 (m, H-5’), 2.99 (dd, J=11.8Hz, J=4.6Hz, H-3), 2.94 (dt, J=7.8Hz, J=4.6Hz, H-2’), 2.14 (o, H-9/18), 1.92 (o, Ha-16), 1.90 (o, Ha-15), 1.87 (o, Ha-11), 1.80 (o, Ha-2), 1.78 (o, Hb-11), 1.58 (o, Ha-1), 1.57 (o, Ha-7), 1.56 (o, Hb-16), 1.54 (o, Hb-2), 1.53 (o, Hb-15), 1.50 (o, 2H-22), 1.44 (o, Ha-6), 1.39 (o, 2H-21), 1.23 (o, Hb-6), 0.94 (s, 3H-23), 0.89 (o, H-19/20), 0.87 (d, J=4.6Hz, 3H-30), 0.88 (s, 3H-26), 0.86 (o, Hb-1/7), 0.81 (d, J=5.3Hz, 3H-29), 0.78 (s, 3H-25), 0.73 (s, 3H-24), 0.6 (d, J=11.4Hz, H-5), 13C NMR (100MHz): 178.3 (C-28), 176.6 (C-27), 132.6 (C-13), 127.9 (C-12), 105.3 (C-1’), 87.9 (C-3), 76.9(C-3’), 76.6 (C-5’), 74.0(C-2’), 70.23(C-4’), 61.26 (C-6’), 55.34 (C-14), 55.26 (C-5), 53.7 (C-18), 47.3 (C-17), 46.2 (C-9), 39.0 (C-10), 38.7 (C-4), 38.6 (C-1), 38.5 (C-20), 36.7 (C-7), 36.5 (C-19), 36.2 (C-8), 35.9 (C- 22), 29.8 (C-21), 27.7 (C-23), 25.6 (C-2), 25.0 (C-15), 24.1 (C-16), 22.4 (C-11), 21.2 (C-30), 18.2 (C-25), 17.9 (C-6), 17.6 (C-29), 16.6 (C-24), 16.1 (C-26).
Compound 7 (3-O-β-D-3'-oxo-glucopyranosyl-ursa-12,20(30)- diene-27,28-dioic acid): Brownish powder (hexane/ethyl acetate 1:1), (HRESI-MS)(-): m/z 643.3492 [M-H]- (calculated for C36H51O10) compatible with C36H52O10, 1H NMR (δppm, CH3OH-d4, 400MHz): 5.63 (m, H-12), 4.68 (s, Hb-30), 4.64 (s, Ha-30), 4.40 (d, J=7.8Hz, H-1’), 4.22 (dd, J=10.1Hz, J=1.7Hz, H-4’), 4.11 (dd, J=7.8Hz, J=1.7Hz, H-2’), 3.92 (dd, J=12.0Hz, J=2.0Hz, Hb-6’), 3,79 (dd, J=12.0Hz, J=4.6Hz, Ha-6’), 3.29 (ddd, J=10.1Hz, J=4.6Hz, J=2.0Hz, H-5’), 3.21 (dd, J=11.5Hz, J=4.5Hz, H-3), 2.32 (d, J=11.8Hz, H-18), 2.26 (o, H-9), 2.25 (o, Ha -16), 2.25 (o, Hb-21), 2.19 (o, Ha-21), 2.1 (o, Ha-15), 1.98 (o, H-19), 1.97 (o, Hb -11), 1.91 (o, Ha -11), 1.90 (o, Hb-2), 1.80 (o, Ha-7), 1.78 (o, Ha-2), 1.77 (o, Hb-16), 1.75 (o, Hb-15), 1.70 (o, 2H-1), 1.67 (o, 2H-22), 1.55 (o, Ha-6), 1.55 (o, Hb-7), 1.37 (o, Hb-6), 1.04 (d, J=6.4Hz, 3H-29), 1.02 (s, 3H-23), 0.99 (s, 3H-25), 0.90 (s, 3H-26), 0.85 (s, 3H-24), 0.77 (m, H-5), 13C NMR (100MHz): 207.55 (C-3’), 180.8 (C-28), 178.8 (C-27), 154.1 (C-20), 133.6 (C-13), 131.08 (C-12), 107.9 (C-1’), 106.2 (C-30), 91.1 (C-3), 78.9 (C-2’), 78.0 (C-5’), 73.8 (C-4’), 62.6 (C-6’), 57.7 (C-18), 57.3 (C-14), 56.9 (C-5), 49.6 (C-17), 48.0 (C-9), 40.7 (C-8), 40.1 (C-4), 40.0 (C-7), 39.9 (C-1), 38.0 (C-22), 37.9 (C-10), 36.7 (C-19), 33.0 (C-21), 28.4 (C-23), 27.0 (C-2), 26.6 (C-16), 25.8 (C-15), 23.9 (C-11), 19.3 (C-6), 19.0 (C-26), 17.0 (C-24), 16.9 (C-25), 16.6 (C-29).
Compound 8 (3-β-(-α-L-rhamnopyranosyloxy)-ursa-12,20(30)- diene-27,28-dioïc acid): Beige powder (ethyl acetate/methanol 9:1), (HRESI-MS)(+): m/z 653.82 [M+Na]+ calculated for C36H54O9, DI=10, 1H NMR (δppm, DMSO-d6, 400MHz): 5.53 (m, H-12), 4.59 (brs, Ha-30); 4.68 (brs, Hb-30), 4.15 (d, J=7.7Hz, H-1’), 3.15 (m, H-5’), 3.07 (dt, J=8.9Hz, J=4.5Hz, H-3’), 2.98 (d, o, H-3), 2.97 (m, H-2’), 2.78 (dt, J=8.9Hz, J=5.4Hz, H-4’), 2.23 (d, J=11.8Hz, H-18), 2.16 (o, H-9), 2.14 (o, 2H-21), 1.99 (o, Ha-15), 1.90 (o, Ha-11), 1.84 (o, H-19), 1.81 (o, Hb-11), 1,75 (o, Hb-2), 1.71 (o, Ha-22), 1.66 (o, 2H-16), 1.61 (o, Hb-15), 1.61 (o, 2H-1), 1.55 (o, Ha-2), 1.55 (o, 2H-7), 1.48 (o, Hb-22), 1.46 (o, Ha-6), 1.25 (o, Hb-6), 1.13 (d, J=6.1Hz, 3H-6’), 0.96 (d, J=6.5Hz, 3H-29), 0.94 (s, 3H-23), 0.89 (s, 3H-25), 0.79 (s, 3H-26), 0.74 (s, 3H-24), 0.61 (o, H-5), 13C NMR (100MHz): 177.7 (C-28), 176.1 (C-27), 152.3 (C-20), 132.3 (C-13), 128.5 (C-12), 105.9 (C-30), 105.2 (C-1’), 87.9 (C-3), 76.6 (C-3’), 75.4 (C-4’), 74.2 (C-2’), 71.2 (C-5’), 55.7 (C-18), 55.3 (C-14), 55.3 (C-5), 47.4 (C-17), 46.16 (C-9), 39.06 (C-8), 38.7 (C-4), 38.55 (C-1), 38.1 (C-22), 36.2 (C-10), 36.2 (C-7), 35.1 (C-19), 31.2 (C-21), 27.7 (C-23), 25.8 (C-2), 25.1 (C-16), 24.1 (C-15), 22.4 (C-11), 18.1 (C-6’), 18.1 (C-26), 17.8 (C-6), 16.5 (C-24), 16.1 (C-25), 16.0 (C-29).
Antioxidant activity
Stem bark, Roots and Leaves crude extracts: This method of measuring antioxidant capacity of a substrate has been validated with Carine’s work that has been used to measure the flavonoids antioxidant capacity [12]. Our results clearly showed that the extracts tested, using this method, showed an antioxidant activity which is quantified for a better assessment by calculating the Antioxidant Index AI30 ((Ipa-Ipa°)/Ipa°=0.30). The stem bark extract is the most active with a lower AI30 of 79.50 mg/L, meaning that a substrate concentration of 79.50 mg/L is required to consume 30% of electrogenerated O2●− while leaves and root crude extracts showed moderate activities with AI30 of 303.70 mg/L and 450.00 mg/L, respectively. Generally in Chadian folk medicine, the stem bark is the most used part of the plant, then powders of stem bark is used as a herbal tea locally called Goub, as well as a hemostatic agent for bloody wounds. From the stem bark extracts, we isolated two saponins, including 3-O-β-D-3'-oxo-glucopyranosyl-ursa-12,20(30)-diene-27,28-dioic acid known for its moderate antibacterial activity [8] and 3β-(-α-L-rhamnopyranosyloxy)-ursa-12,20(30)-diene-27,28-dioic acid isolated for the first time from extracts of C. febrifuga (Figures 2-5).
Figure 2: Linear response of o2.- consumption depending on the stem bark extract concentration.
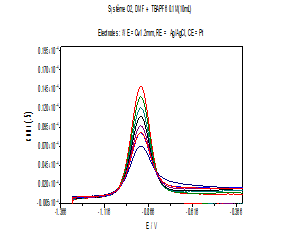
Figure 3: Cyclic voltammograms of O2 reduction in absence and presence of increasing concentrations of stem bark extract at a stable glassy carbon disc electrode in 0.1M (Bu4NPF6) solution. 0.1 V / s scan rate.
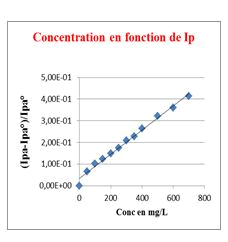
Figure 4: Linear response of O2 consumption as a function of root extract concentration
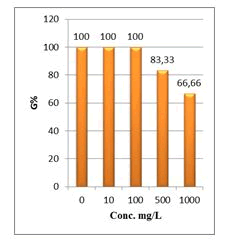
Figure 5: Linear response of O.-2 consumption as a function of leaves extract concentration
Phytotoxicity
Shanzhiside methyl ester (1) and roots crude extracts: Our results revealed that all the tested crude extracts and compound (1) exhibited phytotoxicity on the germination and growth of seeds at varying degrees. Most plants contain inhibiting substances on the seed germination and the degree of inhibition is proportional to the concentration [30]. We found that at a certain concentration, mostly relatively low (10 and 100 mg/L), the extracts have a stimulating effect on growth but become toxic or phytotoxic at high concentrations. Some compounds inhibit seed germination at 50 ppm but stimulate growth below 1ppm [30]. The root extracts are more toxic against seed germination with the germination percentage (G %) ranging from 66.66 to 100% and the relative root growth (RRG %) from 8.20 to 88.45%. From 500 mg/L, the seedlings are stunted with average root and stem lengths of 3.36 and 4.01mm respectively, whereas these values are respectively 41.05 and 13.66 mm for the control (in the absence of extract). In addition to the physiological parameters reduced between 500 and 1000 mg/L, yellowing of the foliage is observed, reflecting the external stress undergone by the seedling. Compound (1), belonging to the iridoids class, stimulated growth between 10-500 mg/L but showed a toxic effect above 500 mg/L. The (G %) values is 95% for the control (0 mg/L), 95% (10 mg/L), 95 (100 mg/L), 85% (500 mg/L), 85% (1000 mg/L) showed that compound (1) have an influence on seed germination while the (RRG %) ranging from 48.72 to 113.82 showed also negative allelopathic effect on seed germination at high concentrations (≥ 500mg/L). Iridoids are compounds originally isolated from Iridomirmex genus ants in Australia and are involved in the defense mechanism of these ants. Iridoids, are known to have a wide range of biological activities including antibacterial, antispasmodic, and antioxidant properties [31]. We evaluated in this study for the first time the phytotoxicity of an iridoid compound, Shanzhiside methyl ester (1) (Figures: 6-13).
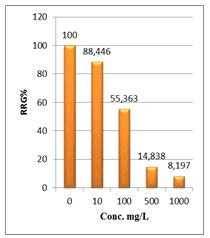
Figure 6: Percentage of germination (G%) of Lepidium sativum seeds exposed to different concentrations of root crude extracts
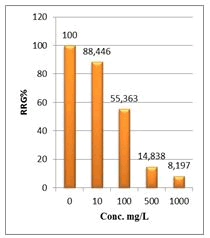
Figure 7: Relative root growth (RRG%) of Lepidium sativum exposed to different concentrations of root crude extracts
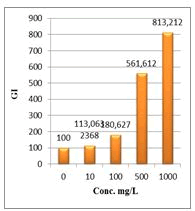
Figure 8: Germination Index (GI %) of Lepidium sativum exposed to different concentrations of root crude extracts
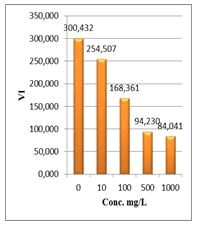
Figure 9: Vigor index (VI) of Lepidium sativum exposed to different concentrations of root crude extracts
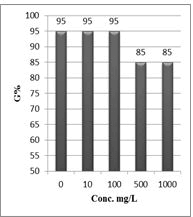
Figure 10: Percentage of germination (G%) of Lepidium sativum seeds exposed to different concentrations of compound (1)
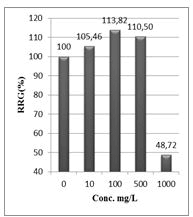
Figure 11: Relative root growth (RRG %) of Lepidium sativum exposed to different concentrations of compound (1)
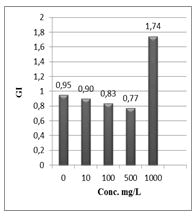
Figure 12: Germination index (GI%) of Lepidium sativum exposed to different concentrations of the compound (1).
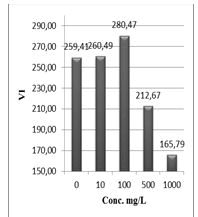
Figure 13: Vigor index (VI %) of Lepidium sativum exposed to different concentrations of the compound (1).
Phytochemical study of crude extracts from C. febrifuga led to the isolation and structural identification of nine compounds belonging to four classes of organic compounds (iridoids, saponins, triterpenes, and sugars). We report the direct isolation of a saponin (3β-(-α-L-rhamnopyranosyloxy)-ursa-12,20(30)-diene-27,28-dioicacid) previously obtained by acid hydrolysis of 3β-(α-L-rhamnopyranosyloxy)-28-O-(β-D-glucopyranosyl)urs-12,20(30)-diene 27, 28-dioïc acid isolated from root extracts of C. febrifuga. The tested compound (1), an iridoid, stimulated seedling growth at low doses (10 mg/L-100 mg/L) and exhibited moderate phytotoxicity at 500 mg/L. The stem bark extract was the most effective against the superoxide anion in electrochemical measurements with an AI30 of 75.9 mg/L. This study completes the wide range of biological activities of C. febrifuga extracts as well as an iridoid compound.
The authors are grateful to Mrs. Geneviève Pillet (Switzerland), Mrs. Arlette Mermier (France), Mr Michel Blanc (France), Mrs. Ginette Blanc (France), Mrs. Danielle Blanc (France), and Mr Bealem Ferdinand (Chad) for supporting this work through a financial help to Bealem Aristide. We also thank Mr. Philippe Jehan (CREMPO, University of Rennes 1) for having carried out mass spectra experiments and for his advices.
Published: 14-Oct-2021, DOI: 10.35248/2329-6836.21.9.416
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.