Research Article - (2020) Volume 8, Issue 1
Objective: The aim of the present study was to characterize the antimicrobial properties of Acacia Gum Arabia crude extracts and Acacia Gum Arabia aqueous cream against certain selected quality control microorganisms.
Methodology: A serial concentrations were prepared 2.5%, 5%, 10%, 15%, and 20% of Acacia Gum Arabia. An aqueous cream with 5% concentration of Acacia Gum Arabia were prepared and evaluated. A quality control tests were conducted to check the stability, organoleptic properties and pH of the cream. An in vitro evaluation against Gram positive bacteria (Bacillus subtilis and Staphylococcus aureus), Gram negative bacteria (Escherichia coli, and Pseudomonas aeroginosa) and yeast (Candida albicans). The Minimum Inhibitory Concentration (MIC) were determined.
Results: Out of sex (6) tested microorganisms, it is found that 5% is the optimum concentration that most of the microorganisms are reported to shows zone of inhibitions. A further experiments were conducted for minimum inhibitory concentrations (MIC). The MIC values are found to be Proteus 1.25%, Shigella 2.5-1.5%, Staphylococcus epidermidis 1.25%, and Klebsiella 2.5%. The quality control tests for Acacia Gum Arabia cream reveals that for the spreadability test the measured diameter of each types (spreadability), in which the average diameter of aqueous cream and acacia cream ware 2.4 and 2.2 ± 0.14, respectively. The topical treatment with Acassia gum cream showed significant healing effect on excision wounds and demonstrated an important role in the inflammation process by increasing antioxidant enzyme activities, thereby accelerating the wound healing process and reducing tissue injury.
Conclusion: it can be concluded that Acacia Gum Arabia demonstrate an antibacterial activity for certain types of bacterias. And the Acacia Gum Arabia cream can be used as an antibacterial cream.
Acacia Gum Arabia aqeous cream; Cream quality control; Disc diffusion method; and Minimum Inhibitory Concentrations (MIC)
Acacia Gums, also known as Gum Arabic (GA), is one of the most commonly used product for a wide range of applications in different pharmaceutical dosage forms. GA is a sap (a fluid) found in the vacuoles (small cavities) from the branches of Acacia trees) [1]. There are a variety of GA species around the world. The gum is usually collected manually after exposing the tree to stressful conditions like injury and draught (Figure 1).

Figure 1: Picture of the Acacia in this project.
Chemically, Acacia gum mainly contains a mixture of ingredients of calcium, magnesium and potassium salts of arabic acid which is named as arabin [2]. GA is a slightly acidic compound, mainly consist of polysaccharide and glycoprotein and their magnesium, calcium and potassium salt [3] polysaccharide backbone is composed of 1,3-linked beta-Dgalactopyranosyl units. The side chains are composed of two to five 1,3-linked beta-D-galactopyranosyl units, joined to the main chain by 1,6-linkages [4,5].
GA has been proven to be an effective emulsifier, binder and stabilizer agent [6] One of the pharmaceutical application is working as a natural emulsifier, to keep ingredients together which normally could not be mixed well. GA can be also used as a binder in tablet dosage forms to hold the pharmaceutical active and inactive ingredients in a cohesive mixture [4]. Moreover, GA possesses various pharmacological uses in different diseases, such as, alleviation of constipation by improving bowel movement, an anti-obesity agent and inducer of satiety and induction of immune system as probiotic substance, which could promotes the growth of normal flora of the gut [6].
GA has demonstrated an anti-bacterial effect against the growth of S. aureus organism which is a known cause of diarrhea [7]. GA has also showed a beneficial effects in treating Acne due to its an anti-microbial properties which can help in curing Acne, especially when combined with other medications, such as Tetracycline or vitamin A [8]. GA has been also used topically as wound healing [9]. Furthermore, Gum acacia has exhibited antimicrobial activities against several gram positive and gram negative bacteria, such as Staphylococcus aureus, Streptococcus viridians, Escherichia coli and Salmonella typhi [10].
Objectives of the study
Aim of the study: The main objective in this study is to evaluate the anti-microbial activities of an aqueous cream prepared from Gum Acacia.
Specific objectives:
1.To determine the quality control measurements of the stability of the aqueous cream.
2. To evaluate anti-microbial activities of the prepared GA cream.
The materials and methods needed for this project is divided in to four major phases according to the laboratories activities as shown in Figure 2.
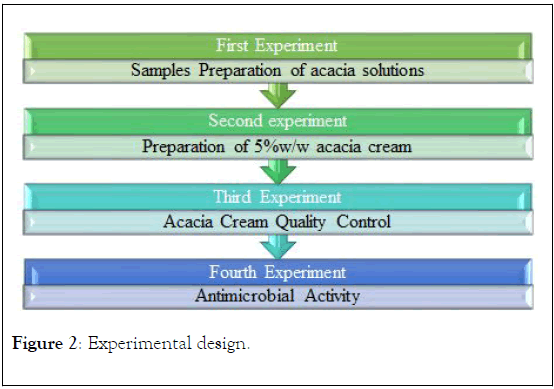
Figure 2: Experimental design.
Samples preparation of Acacia solutions
In order to prepare 20% w/v acacia solution, 20 g of Arabic gum (Figure 3) was dissolved in 100 mL of distilled water (in a glass beaker covered with parafilm) using magnetic stirrer (Thermo Scientific©, Model: SP 131320-33) at room temperature as showed in (Figure 3A). Then, the result solution (Figure 3B) was utilized to prepare four concentrations (10%, 7.5%, 5%, and 2.5% w/v acacia solutions) for testing antimicrobial activity (Figure 3C).
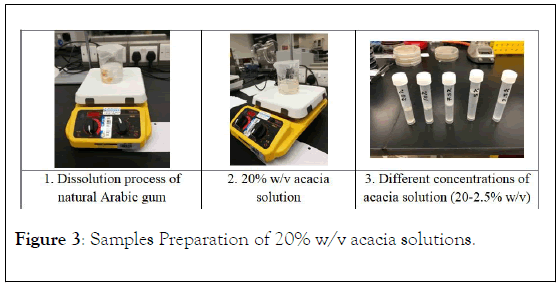
Figure 3: Samples Preparation of 20% w/v acacia solutions.
Preparation of 5%w/w Acacia cream: Oil in water (O/W) emulsion-based cream will be formulated according to the method stated [11-18]. The emulsifier (Gum Arabic) and other oil soluble components (Cetyl alcohol, and almond oil) will be dissolved in stainless steel bowl in a water bath to form the oil phase (Part A) which finally heated to 93°C. The water soluble components (Methyl paraban, Propyl paraban, Triethanolamine, and Propylene glycol) will be dissolved in the aqueous phase (Part B) and heated to 75°C. After heating, the aqueous phase will be added in portions to the oil phase with a continuous stirring until cooling of the emulsion take place [19,20].
All ingredients were melt together at 93°C with continuous stirring until cooled down to room temperature as shown in Figure 4. As described in the below (Table 1).
Then, 60 g of Emulsifying Wax was utilized to prepare 200 g of Aqueous Cream BP.
| Ingredient | Quantity to prepare 60 g |
|---|---|
| Emulsifying wax | 18 g |
| White soft paraffin | 30 g |
| Liquid paraffin | 12 g |
Table 1: Ingredients and their quantities to prepare 60 g.

Figure 4: Preparation of solution A.
Preparation of Aqueous Cream BP: As described in Table 2 below, Formula 2 (200 g).
The Phenoxyethanol was dissolved in 138 g of the purified water at 60°C to produce solution A. In a separate bowl, 60 g of Emulsifying Ointment was also melted at 60°C to produce mixture B (Figure 4).
| Ingredient | Quantity to prepare 200 g |
|---|---|
| Emulsifying ointment | 60 g |
| Phenoxyethanol | 2 g |
| Purified water, freshly boiled and cooled q.s. to make | 200 g |
Table 2: Formula 2 (200 g).
Finally, solution A was added gradually to mixture B with continuing stirring at the same temperature (60°C) until cooled. Then the mixture was prop sonicated (QSonica 700 prop sonicator) for 1 minute (intensity 40%) to produce the final cream as shown in Figure 5.
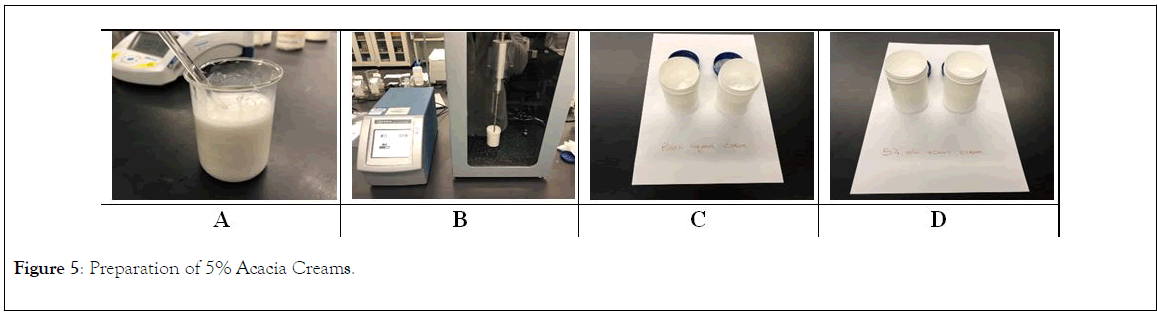
Figure 5: Preparation of 5% Acacia Creams.
The 5%w/w acacia cream (10 g/200 g cream) was produced by incorporation of acacia in the aqueous phase (solution A).
Acacia cream quality control
Organoleptic evaluation: Both blank (aqueous) and acacia creams were tested for general appearance, color, homogeneity, and phase separation. General appearance, color, and phase separation were evaluated by visual observation. For homogeneity, small quantity (around 1 g) of cream was squeezed (pressed) between two fingers in order to determine the degree of greasiness as well as presence of any coarse particles.
Spreadability: The spreadability is a very important factor of any semi-solid formulation to determine the covered area per specific weight of the formula. The spreadability of both creams was evaluated by measuring the spreading area of the cream (1 g) between two glass plates (20 cm × 20 cm) after 1 minute of applying standard weight of 50 g.
Determination of pH: From each type of cream (aqueous and acacia creams), 2 g was dispersed in 50 mL of deionized water. Then, the pH value of each result solutions was measured in triplicate using a calibrated pH meter (Thermo Scientific A211).
Antimicrobial activity
Disc diffusion method: The in vitro antimicrobial activities was carried out using the disc diffusion method [13-19]. The diffusion technique is suitable for testing aqueous suspensions of plant extracts, since the presence of suspended particles in the sample being tested is less likely to interfere with the diffusion of the antimicrobial substance into the agar than other methods, such as the filter paper disc and cylinder plate systems. Nutrient Agar (NA) medium sterilized and poured into the plate; and allowed to harden [9].The surface of the NA plate was then be inoculated with a sterile swab of the selected microorganism namely Staphylococcus aureus, Escherichia coli, Staphylococcus epidermidis, Streptococcus agalactiaea and Pseudomonas aeroginosa. The discs containing anti-microbial agent (namely tetracycline, gentamycin and ampicillin) were used as controls. Discs were loaded with 10 μl of various concentrations of acacia solutions and arranged on the surface of the inoculated plates in such a way so as to be at least 20 mm apart and incubated at 35°C-37°C for 18-25 hours. The plates then were examined for the presence of inhibitory zones and the diameter of the inhibition zone was measured [20,21].
Microtitre plate for Minimum inhibitory concentration (MIC): Microdilution test was also used to test for the antibacterial activity of acacia solutions. Serial dilution of each concentration was performed on 96-well microtitre plate containing 100 μl nutrient broth. Wells were inoculated with bacteria, incubated at 35°C-37°C for 24 hours. After incubation, turbidity was observed in the inoculated wells and compared to the control wells. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were recorded. Each assay was performed in duplicate.
Wound healing activity
Experimental design: Four groups of an adult female SD rats were arbitrarily divided, with five rats in each group. Each rat was stored separately. The rats were sedated. An electrical shaver was used to shave the skin and disinfected with 70% isopropanol. A uniform wound area with 2.5 cm diameter was excised from the back side of the neck of the rats using a round seal as shown in Figure 6. Incision of the muscle layer was avoided, and skin tension was kept constant during the procedure. All rats were treated twice a day, as follows: the control group was left untreated; the reference drug group was treated with 0.2 mL intrasite gel; one of the treated groups was topically administered 0.2 mL of the 100 mg/mL plant extract; and the other treated group was topically administered 0.2 mL of the 200 mg/mL plant extract. Contraction of the wound area was measured at days 0, 4, 7, and 14 after incision. At day 14, a high dose of anesthesia was administered to all the experimental animals, and the skin from the healed wound area was excised in order to obtain a homogenous tissue for histopathological examination. Blood samples were also collected to measure other parameters [16].
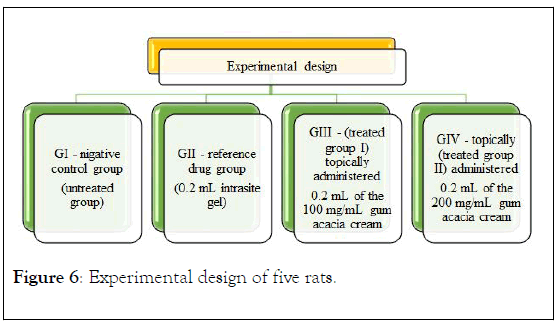
Figure 6: Experimental design of five rats.
Histological evaluation of wound healing: A piece of skin from the healed wound area was fixed in 10% formalin and then dehydrated with increasing alcohol concentrations in an automated tissue processing machine. The processed tissue was embedded in paraffin for tissue sectioning. A microtome was used to cut the tissue into 5 μm sections for staining with hematoxylin – eosin solutions and Masson ’ s Trichrome stain according to standard protocols [17].
Acacia cream quality control
Organoleptic evaluation: Both types of creams are opaque, white, homogenous, and there is no phase separation in both types. Upon application on the skin, there is no coarse particles and they are no greasy effect after drying of the cream on the skin surface.
Spreadability: After 1 minute of the test, the area of the tested cream was measured by a micrometer in order to determine the diameter of the circle formed by the cream. Figure 7 showed the measured diameter of each types (spreadability), in which the average diameter of aqueous cream and acacia cream were 2.4 and 2.2 ± 0.14, respectively.
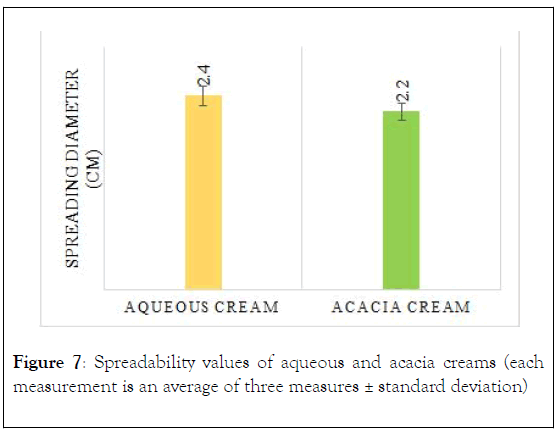
Figure 7: Spreadability values of aqueous and acacia creams (each measurement is an average of three measures ± standard deviation)
Determination of pH: A three pH measures were done for the 5% acacia aqueous solutions, and 5% acacia cream. The mean pH of the two samples were found to be pH of aqueous cream=5.54 pH of acacia cream=6.21.
Antimicrobial bioactivity
Disc diffusion method: After inoculating the surface of the NA plate with a sterile swab of the selected microorganism mentioned previously. The discs containing anti-microbial agent (namely Tetracycline, Gentamycin and Ampicillin) were used as +Ve controls. Discs were loaded with 10 μl of various concentrations of acacia solutions and arranged on the surface of the inoculated plates in such a way so as to be at least 20 mm apart and incubated at 35°C-37°C for 18-25 hours. The plates then were examined for the presence of inhibitory zones and the diameter of the inhibition zone was not able to be measured, (Figure 8). The antimicrobial activity was represented in a form of -ve for the microorganism which shows no ZOI, and +ve were used to demonstrate that it shows ZOI at a specific concentration to be as explained in the below Table 3. Each experiment were repeated in triplicate.
| Microorganism | Zone of Inhibition detection detection at specific concentration |
|---|---|
| Staphylococcus aureus | -ve |
| Staphylococcus epidermidis | +ve at 10% |
| Escherichia coli | -ve |
| Streptococcus agalactiaea | -ve |
| Pseudomonas aeroginosa | -ve |
| Shigella | +ve at 5% |
| Klebsiella pneumoniae | +ve at 5% |
| +ve: means zone of inhibition were detected -ve: means zone of inhibition were not detected |
|
Table 3: Detection of Zone of Inhibition in a serial of concentrations (%) 2.5, 5, 7.5, 10, and 20.
Figure 8: Agar plates were inoculated with several microorganisms.
Microtitre plate for Minimum inhibitory concentration (MIC): On a serial dilution of each concentration that was performed on 96-well microtitre plate, that incubated for 24 hours. After incubation, turbidity was observed in the inoculated wells usnig microtitre plate reader, and compared to the control wells. Minimum inhibitory concentration (MIC) is recorded. Each assay was performed in duplicate. The inoculation results were microorganisms are Proteus, shigella, represnted in Figure 9A-9D to demonstrate different Staphylococcus epidermidis, and Klebsiella pneumoniae respectively [22-26].
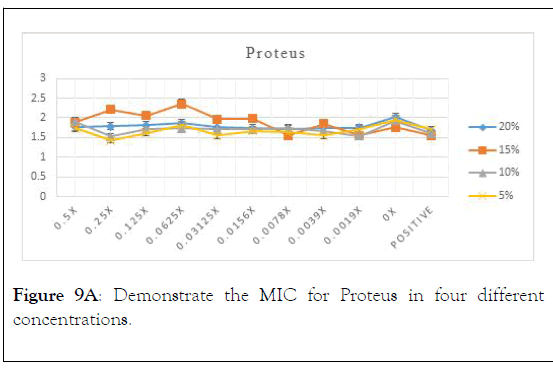
Figure 9A: Demonstrate the MIC for Proteus in four different concentrations.
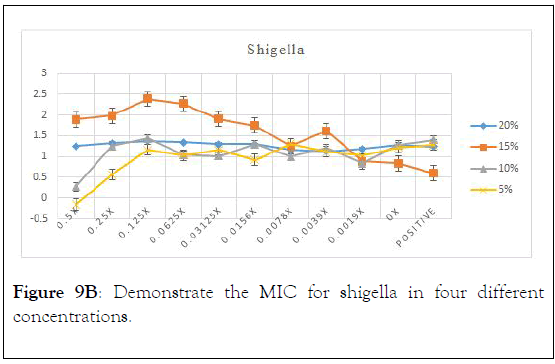
Figure 9B: Demonstrate the MIC for shigella in four different concentrations.
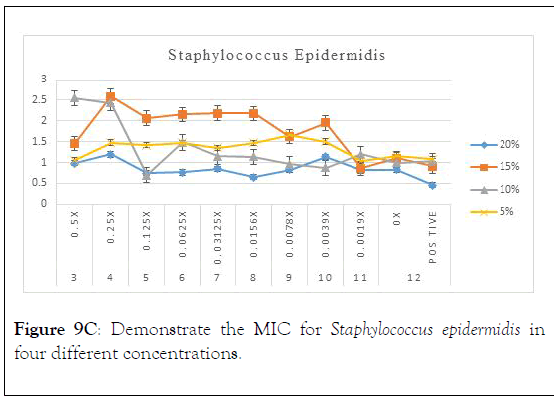
Figure 9C: Demonstrate the MIC for Staphylococcus epidermidis in four different concentrations.
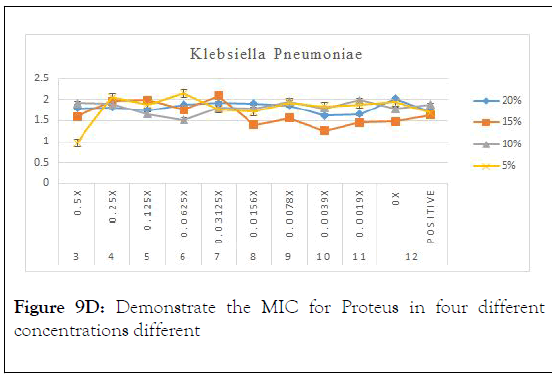
Figure 9D: Demonstrate the MIC for Proteus in four different concentrations different
As in the above charts areas it is found that 5% is the best concentration that shows minimum inhibitory concentration with proteus. It is also showed that 0.25 is the optimum serial dilution that shows least turbidity.
As in the above charts areas it is found that 5% is the best concentration that shows minimum inhibitory concentration with shigella. It is also showed that from 0.5 to 0.125 is the optimum serial dilution that shows least turbidity.
As in the above charts areas it is found that 20% is the best concentration that shows minimum inhibitory concentration with Staphylococcus epidermidis. It is also showed that 0.125 is the optimum serial dilution that shows least turbidity. We can comment also that Staphylococcus epidermidis has shown inhibition on the turbidity with more concentration which could be due to more viscosity.
As in the above charts areas it is found that 5% is the best concentration that shows minimum inhibitory concentration with Klebsiella pneumoniae. It is also showed that 0.5% is the optimum serial dilution that shows least turbidity. Conclusion of the above results is in Table 4.
| Microorganism | MIC |
|---|---|
| Proteus | 1.25% |
| Shigella | 2.5 – 1.5% |
| Staphylococcus epidermidis | 1.25% |
| Klebsiella | 2.50% |
Table 4: MIC of different microorganisms.
Wound healing activity
Wound contraction: Results from the macroscopic evaluation of wound healing rate on day 15 after incision revealed that the wounds dressed with P. macrocarpa showed considerable signs of dermal healing and significantly healed faster compared with the vehicle-treated control group (gum acacia in normal saline) as presented in Figure 10. A measurement of wound healing progression showed that the plant extract induced wound healing (Figure 2). An excision wound margin was traced after wound creation using a transparent paper, and the wound area was measured using a graphing paper. Wound contraction was measured at 5-day-intervals until day 15. In the excision wound model, the wounds of the animal groups treated with 100 and 200 mg/mL P. macrocarpa fruit extract contracted by 25.6 ± 10.8 and 19.2 ± 8.7 mm2, respectively, at significantly higher rates at day 15 (p ≤ 0.05) compared with the vehicle-treated control group (55 ± 10.3 mm2).
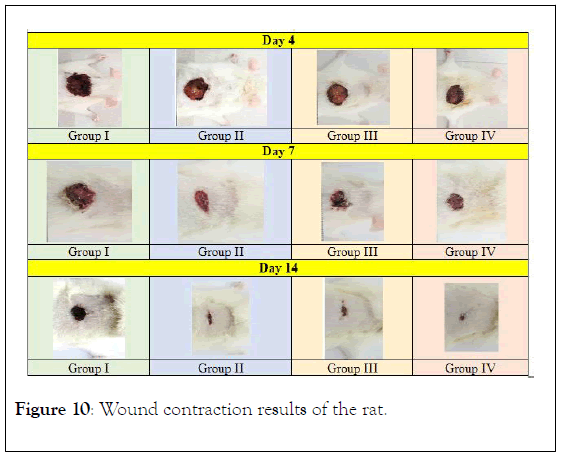
Figure 10: Wound contraction results of the rat.
Histopathological evaluation of healed wound area: The histology of the wound tissues on the 15th day after wounding was calculated. Wound enclosure was found to be smaller in the acassia gum extract-treated groups (Figure 11) and the granulation tissues contained comparatively less inflammatory cells and more collagen, fibroblast, and blood proliferating capillaries than the -ve control group, as revealed by Masson’s Trichrome staining.
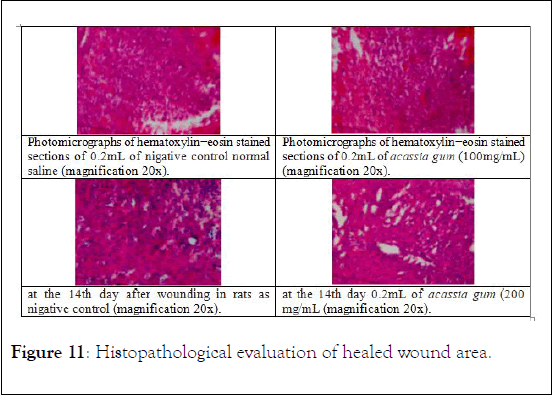
Figure 11: Histopathological evaluation of healed wound area.
MIC is the minimum inhibitory concentration of antibiotic (plant) that prevent the bacteria to grow any more, Out of sex (6) tested microorganisms, it is found that 5% is the optimum concentration that most of the microorganisms are reported to shows zone of inhibitions. A further experiments were conducted for minimum inhibitory concentrations (MIC). The MIC values are found to be Proteus 1.25%, Shigella 2.5 – 1.5%, Staphylococcus epidermidis 1.25%, and Klebsiella 2.5%. The quality control tests for Acacia Gum Arabia cream reveals that for the spreadability test the measured diameter of each types (spreadability), in which the average diameter of aqueous cream and acacia cream ware 2.4 and 2.2 ± 0.14, respectively.
As a Conclusion it can be concluded that Acacia Gum Arabia demonstrate an antibacterial activity for certain types of bacterias. And the Acacia Gum Arabia cream can be used as an antibacterial cream.
Topical treatment with acassia gum cream improved the activities of wound healing by reducing cellular damage and accelerating the wound healing process.
Citation: Suliman RS, Ali H, Alamer A, Aleid N, Abu-Jafal A, Abdulgadir R, et al. (2020) In vitro Anti-microbial Activity and Wound Healing Evaluation of Acacia Gum Arabia Aqueous Cream. Nat Prod Chem Res. 8:369. DOI: 10.35248/2329-6836.20.8.369
Received: 03-Feb-2020 Published: 24-Feb-2020, DOI: 10.35248/2329-6836.20.8.369
Copyright: © 2020 Suliman RS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.