Case Report - (2024) Volume 13, Issue 1
Ventricular Assist Devices (VAD) are blood pumps that can stabilize the hemodynamics of patients with severe heart failure. The VAD indication is for therapeutically assist before, during and/or after transplant, recovery and destination therapic. Our group propose a centrifugal blood pump as VAD that consists of a rotor magnetically coupled, suspended by pivot bearings. The pivot is mainly a ceramic axis with rotational movement and the bearing is a polished surface made mainly with polymers. As a whole system, a VAD should be made of long-life biomaterials to avoid degradation or deformation during application lifetime. Surface modification techniques have been widely studied and implemented to improve properties such as biocompatibility and durability of applicable materials. The chemical vapour deposition technique allows substrates having melting point higher than 300°C to be coated, encapsulated, with a Diamond Like Carbon (DLC) film. The amorphous carbon film coated material provides the surface properties such as hardness and improved self-lubricating and biocompatibility for specific application. The objective is to study VAD pivot bearings made PEEK and medical grade titanium with DLC coating. Regarding VAD continuous operation and its relation with the patient life support, the system must have a high level of reliability. In order to evaluate and compare the performance, two other pivots were made in titanium c.p. and alumina ceramic. The bearing was made of PEEK for each pivot. Then, there was the physical characterization of each component by electron microscopy and volume control, before and after in vitro tests of durability. The tests simulated the VAD friction and wearing. The results showed less deformation by abrasive wear and promising alternative to use DLC in VAD pivot bearings.
Hemodynamics • Pivot bearings • Biocompatibility • DLC coating
Cardiovascular diseases are the world leading cause of death, accounting for approximately 30 percent of global deaths. An Implantable Centrifugal Blood Pump (ICBP) was designed for long term circulatory support in cardiac patients. Design was focused in computational simulations compared with empirical development that is historically common in artificial organs field of study. Computational simulations avoid unnecessary costs and promote deeper knowledge of involved phenomena. However, different situations had been studied and simulated with isolated dynamic models making difficult to couple solutions. The empirical development of mechanical circulatory support using animal experiments can be traced back to 1930’s. Until the first human clinical application of Ventricular Assist Devices (VAD) in 1966, engineering was not able to apply mathematical models and numerical simulations to design blood pumps and propose precise solutions to substitute the natural heart to an artificial organ [1]. The Implantable Centrifugal Blood Pump (ICBP) described in this paper is a VAD that can promote long term circulatory support in cardiac patients, Figure 1. Our group started this project in 2006 and now it is part of a multicenter study with associated researchers in more than 6 laboratories.
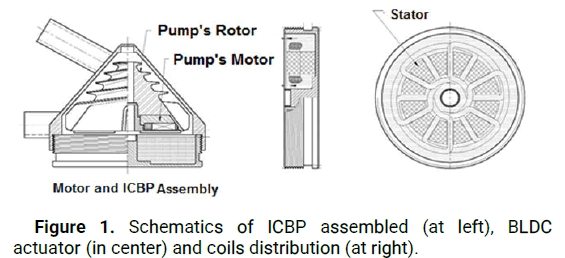
Figure 1: Schematics of ICBP assembled (at left), BLDC actuator (in center) and coils distribution (at right).
The components are made in biomaterials such as medical grade titanium; with the exception of the pivot bearing that must be made in biomaterials high durability for testing and interaction of applications with blood. Bock et al. studied the wear of various combinations of biocompatible materials of pivots and bearings for application to centrifugal blood pumps; in this study, he concluded that the bearing combination of in ceramic alumina with pivots polymer of ultra-high molecular weight polyethylene shows less abrasive wear during the actuation of prototype, where the rotor reaches about 3000 rpm to generate flow of 5 L/min to 100 mmHg.
Because of the need to increase reliability, biocompatibility and useful life of implantable medical devices, in particular VAD's; the pivot bearing system and the relevant biomaterials are being studied again to support a minimum application time of 10 years without showing action of deterioration or deformation [2].
A bearing consists of the inner part, the pivot usually cylindrical and rotary motion and the fixed bearing surface, the bearing. Smooth surfaces are ideal for such applications since if irregularities certain spots of the surface may be closer to one another causing the lubricant does not play a part in friction conditions between the surfaces and the fluid; excessive friction between the surfaces leads to abrasion of the material with lower hardness and consequently the lower life of the system; in VAD's, this condition will lead to excessive breakdown of red blood cells, hemoglobin release in the blood plasma; i.e, high rate of hemolysis.
The prediction of the abrasive wear rate is controlled by plastic deformation rate and is presented from the Archard equation originally developed for the sliding wear. The suitability of equation 1 for abrasive wear due to Rabinowiczes; where the worn abrasive penetrates the surface at a depth that is proportional to the ratio between the hardness and applied force.
The formed groove volume is completely removed and released into the circuit; there was thus forming the angle of attack as the geometry of the abrasive [3].
The technique Chemical Vapour Deposition (CVD) has been applied to modify surfaces of certain materials to improve their properties depending on the condition and the time of application. The coating surfaces with Diamond Like Carbon film (DLC) by CVD is used increasingly in medical and spatial areas to customize materials subjected to physical stress, chemical, thermal or radiation for a long period. The DLC film is a metastable form of amorphous carbon containing a significant fraction of sp3 type bonds; its properties suggest high mechanical hardness, chemical stability, high resistance to wear and corrosion and it is also biocompatible; pure DLC also has a bactericidal activity of 30% against Escherichia coli.
Here we present the result of a collaborative effort between the Center Engineering in Assistance Circulatory (CEAC) of IDPC and the Laboratory Associated of Sensors and materials (LAS) of Institute National for Space Research (INPE). The components of the pivoting support system of VAD's centrifugal in different materials, uncoated and coated with DLC, have been tested In vitro durability to select, in a comparative way, the pivoting assembly that have lower wear rate [4].
The making of pivots and bearings of the constituents of the pivoting support system was held at the engineering center for circulatory assistance through conventional machining technique.
It was used Polyether Ether Ketone (PEEK) to fabricate four support surfaces, bearings, for each pivotal in study; pure titanium grade medical for 2 pivots, PEEK for 1 pivot. The technique Plasma Enhanced Chemical Vapor Deposition (PECVD), it was applied to coat 2 pivots with DLC, 1 in titanium and the other in PEEK at INPE (Figure 2) [5].

Figure 2: Bearings in PEEK left; followed by pivot in titanium with DLC, pivot in PEEK with DLC and pivot in pure titanium.
The DLC film was produced from methane (CH4) and DC pulsed technique PECVD consisting of a discharge in a low pressure plasma using a switching power supply pulse for the generation of plasma and deposition of DLC films on substrates.
The pivot in alumina used in the studies, was also adopted. This study consisted of 4 tests In vitro of durability carried out identically; Table 1 shows the sequence of the tests and the pairs of adopted materials [6].
| Tests | Pivot | Bearing | |
|---|---|---|---|
| 1 | Titanium with DLC | X | PEEK |
| 2 | PEEK with DLC | X | PEEK |
| 3 | Titanium medical | X | PEEK |
| 4 | Alumina | X | PEEK |
Table 1. Pivot bearing sets ordered for testing in vitro durability.
The simulated test for 6 hours uninterrupted actual circumstances in which the support system remains during application on a pivoting VAD to select the set that has less wear or deformation [7]. The test bench for the In vitro durability test was composed of a digital scale, a milling machine (LFG, X63250) a PEEK bearing, a pivot, a support for the pivot and a lubricating solution (1/3 glycerin+1/3 ethanol 70%+water) to simulate the density and viscosity of blood. The wear rate, i.e. the volumetric loss on distance or milligrams per meter (mg/m) of the material with lower hardness was determined by comparative statistical methods applying results of macroscopic and microscopic dimensional analysis and by estimating volume of the bodies before and after the tests. The density of the bearings and the pivots was recorded in milligrams for a digital precision scale (Adventurer, OHAUS) in IDPC; the micrograph of the surface was obtained by an electron microscope MIRA3-EGF Scan (TESCAN) at INPE [8].
The presence of carbon nanocrystals with typical morphology of DLC nucleation "cauliflower" on the surface of the PEEK polymer can be confirmed by micrographic analysis FEG MIRA3 to 5 um resolution in Figure 3 [9]. Table 2 shows the weight values estimated by a precision balance, in milligrams, of each component under study; analyzes were performed before and after the bodies were subjected to in vitro assay [10]. The micrographs of the surface under study revealed the impressive remains and traces resulting from abrasive wear; Figure 4 displays some aspects studied the PEEK bearing surface; before being subjected to wear and after in vitro assay [11].
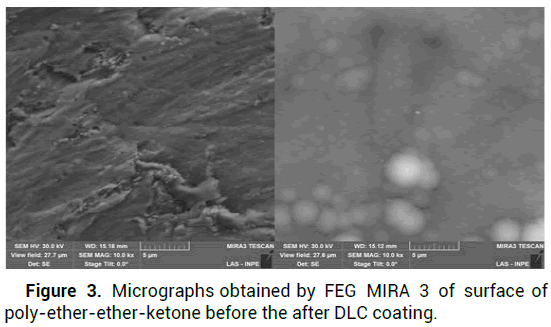
Figure 3: Micrographs obtained by FEG MIRA 3 of surface of poly-ether-ether-ketone before the after DLC coating.
| Ensemble | Component | Material | Mass early (mg) | Mass Final (mg) | Massa Equivalent (mg) |
|---|---|---|---|---|---|
| 1 | Bearing | PEEK | 186.7 | 186.3 | 0.4 |
| Pivot | Titanio puro | 523.2 | 523 | 0.2 | |
| 2 | Bearing | PEEK | 166.3 | 166.1 | 0.2 |
| Pivot | Titanio c/DLC | 915.2 | 915.1 | 0.1 | |
| 3 | Bearing | PEEK | 182.2 | 182.0 | 0,2 |
| Pivot | PEEK c/DLC | 145.5 | 145.4 | 0.1 | |
| 4 | Bearing | PEEK | 189.6 | 189.2 | 0.4 |
| Pivot | Alumina | 178.84 | 178.81 | 0.03 |
Table 2. Volume of mass of bodies in studies.
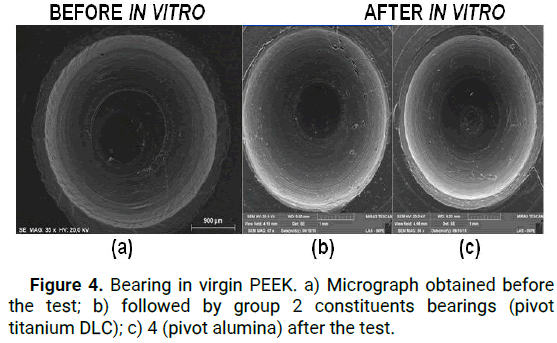
Figure 4: Bearing in virgin PEEK. a) Micrograph obtained before the test; b) followed by group 2 constituents bearings (pivot titanium DLC); c) 4 (pivot alumina) after the test.
The PECVD technology proposed in this work was for maximize the durability of the pivotal system failure prevention by BSCI on the surface by abrading. The well adhered DLC coating tends to make the pivots on the surface smoother pivotal system with greater hardness and titanium; such properties are very promising for plain bearings, since if there are irregularities on the surface of a pivoting system, certain points may be closer to one another and the lubricant, in this case, blood cannot play its role in conditions friction. Consequently, the biofunctionality the device is compromised and the normalized hemolysis index will be high and unsuitable for VAD's [12,13].
The set 4, PEEK bearing and pivot alumina was the group that presented lower mass loss of the abrasive component, 0.03 mg; therefore, future studies should be made bearing finish PEEK with DLC to make a new durability test in order to reduce the mass loss of the bearing; as in the tests in conjunction with the pivot coated with DLC, the PEEK bearing showed a lower weight loss of 0.2 mg. An in vitro interaction assay with blood must also be done in future studies considering the entire system of VAD; because we know the damage to blood cells by the mechanical action of the pivot system and the degree of toxicity of the particles released into the circuit by abrasive wear.
The authors would like to thank the Associated Laboratory of Sensors and materials (LAS) of National Institute for Space Research (INPE) and Brazilian funding agencies FAPESP, CAPES and CNPq.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Bock EGP, et al. "First Studies with a DLC Pivot Bearing for Ventricular Assist Devices". Reconstr Surg Anaplastology, 2024,13(1), 1-3.
Received: 06-Jul-2020, Manuscript No. ACR-24-5312; Editor assigned: 09-Jul-2020, Pre QC No. ACR-24-5312 (PQ); Reviewed: 23-Jul-2020, QC No. ACR-24-5312; Revised: 15-May-2024, Manuscript No. ACR-24-5312 (R); Published: 12-Jun-2024, DOI: 10.37532/acr.24.13.1.001--003
Copyright: © 2024 Bock EGP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.