Research Article - (2024) Volume 12, Issue 1
The aim of this study was to extract essential oil from selected medicinal and aromatic plant leaves and flowers for natural perfume formulation. Fragrant essential oils were extracted from L. angustifolia, C. martini, R. officinalis, C. nardus, B. papyrifera, J. procera and T. schimperi by using steam distillation and sohxlet extraction method and the essential oil yield was 1.3%, 1.04%, 0.91%, 0.83%, 5%, 2.3% and 1.17% (w/w) respectively. The GC-MS result of extracted essential oil analysis revealed there are different compounds in each of the plant extract essential oils that give fragrance scent. Some of the chemical compounds that are found in fragrant essential oil such as linalool, geraniol, citronellol, α- pinene, β-pinene, citronellal and eucalyptol are the main chemical components of perfume scent. Formulation of perfume was done by using fragrance essential oil and ethyl alcohol as a solvent with different proportion of the fragrance oil combination. The panelist hedonic test result, showed that, the first chosen perfume contain, L. angustifolia as top note; Cymbopogon martini as a middle note and Boswellia papyrifera as a base note and the second were Cymbopogon nardus as top note; Rosmarinus officinalis as a middle note and Boswellia papyrifera as base note.
Essential oil • Extraction • Formulation • Fragrance • Panelist • Perfume
The fragrance materials employed in perfumery have natural origin (animal or plant) and synthetic origin or artificial fragrant molecules called aroma chemicals however are chemically identical to their natural counterparts [1].
Essential oils are extracted from the whole aromatic plant or a part of it and although the method of extraction is very important to yield an essential oil capable of producing almost as the raw plant aroma. Perfume is a mixture of fragrant essential oils and aroma compounds, fixatives and solvents used to give the human body, objects and living spaces a pleasant smell. Since the beginning of recorded history, humans have tried to mask or improve their own odor by using perfume which emulates nature's pleasant smells. Perfumes are formulated by adding essential oils (concentrates) incorporated with ethanol and water and some fixatives. Although there is no single "correct" technique for the formulation of a perfume, there are general guidelines as to how a perfume can be constructed from a concept. Eau de perfumes are usually formulated in oils and are normally clear and generally have an amber color due to the natural color of the oils. Perfume composed of 15%-30%aromatic compounds, 90%-95% alcohol and 5%-10% water.
A perfume is a unique mixture of top, middle and base notes designed to give a particular harmony scents. These three notes are categorized as the basics in a perfume. Top notes are small light molecules and make up 15%-25% of the fragrance. Some of top note fragrance source are: Lemon, lime, basil, bergamot, cardamom, clary sage, coriander, eucalyptus, grapefruit, junipers, lavender, lemon grass, mandarin, orange, pine, peppermint, tea tree and thyme. The middle note compounds form the "heart" or main body of a perfume and act to mask the often unpleasant initial impression of base notes which become more pleasant with time and make up of 30%-40% of the total fragrance. Some of natural fragrance used for middle note are cedar wood, cinnamon, clove, geranium, jasmine, marjoram, palma rosa, chamomile, rose. Base notes or bottom notes comprise of 40%-55% of the total fragrance and tend to be long lasting [2].
In Ethiopia there are enormous amount of aromatic plant species, but most of them have not been utilized for the production of valuable product in large production capacity. However they are consumed as traditional medicine and food additives. Some of the aromatic plants are used for only for gardening purpose only. This research open the way to utilization of locally available aromatic plants for preparation of natural perfumery air fresheners and cosmetics additives. According to Ethiopian revenue and custom authority, Ethiopia import perfume an average of 112,589.908 kg annually from different countries to satisfy the demand of the buyers [3].
This research was done in Debre Berhan university, Ankober research center and chemistry department laboratory. Fresh Cymbopogon nardus, Cymbopogon martini, Rosmarinus officinalis, Thymus schimperi, Lavandula angustifolia, Juniperus procera (berries) Foenculum vulgare were collected from Ankober nursery site and Boswellia papyrifera were purchased from Addis Ababa aromatic spices store. The collected plant materials were washed to remove unwanted dust material, crushed cut to smaller size to make easier for the extraction process and stored overnight in shade and essential oils were extracted by steam distillation. Boswellia papyrifera was extracted by solvent extraction (sohxlet extraction method) by using hexane as a solvent (Table 1) [4]. The percentage yield was calculated based on initial mass of a sample and mass of oil obtained using the formula as follows.
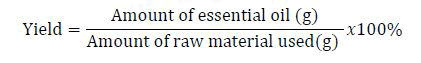
The chemical components found in essential oils were identified by the GC-MS analysis using HP 5890 series GC equipped with Mass Selective Detector (MSD), HP 5972 series (German) in Addis Ababa university, natural science campus.
The essential oils from flowers and leaves were separately blended together according to a formula given by Vankar Padma S. Ethanol (95%ABV) were added to the mixture to homogenize undissolved ingredients [5].
| No. | Top notes | Middle notes | Base note |
|---|---|---|---|
| 1 | L. angustifolia | C. martini | B. papyrifera |
| 2 | C. nardus | R. officinalis | B. papyrifera |
| 3 | J. Procera | T. schimperi | B. papyrifera |
| 4 | L. angustifolia and J. Procera | C. martini. R. officinalis | B. papyrifera |
| 5 | L. angustifolia and J. Procera | C. martini and F. vulgare | B. papyrifera |
| 6 | L. angustifolia and J. Procera | C. martini and T. schimperi | B. papyrifera |
| 7 | L. angustifolia, C. nardus | C. martini. and F. vulgare | B. papyrifera |
| 8 | L. angustifolia, C. nardus and J. Procera | C. martini, T. schimperi | B. papyrifera |
| 9 | L. angustifolia, C. nardus and J. Procera | C. martini, F. vulgare | B. papyrifera |
| 10 | L. angustifolia, J. Procera, C. narche | C. martini, T. schimperi | B. papyrifera |
| Prepared mixture with similar composition treated with 35°C | |||
| 11 | L. angustifolia | C. Martini | B. papyrifera |
| 12 | C. nardus | R. officinalis | B. papyrifera |
| 13 | J. procera | T. schimperi | B. papyrifera |
| 14 | L. angustifolia and J. Procera | C. martini, R. officinalis | B. papyrifera |
| 15 | L angustifolia and J. Procera | C. martini, and F. vulgare | B. papyrifera |
| 16 | L. angustifolia and J. Procera | C. martini and T. schimperi | B. papyrifera |
| 17 | L. angustifolia, C. nardus | C. martini and F. vulgare | B. papyrifera |
| 18 | L. angustifolia, C nardus and J. Procera | C. martini, T. schimperi | B. papyrifera |
| 19 | L. angustifolia, C. nardus and J. Procera | C. martini, F. vulgare | B. papyrifera |
| 20 | L. angustifolia, J. Procera, C. narche | C. martini, T. schimperi | B. papyrifera |
| Prepared mixture with similar composition treated with 45°C | |||
| 21 | L. angustifolia | C. Martini | B. papyrifera |
| 22 | C. nardus | R. officinalis | B. papyrifera |
| 23 | J. procera | T. schimperi | B. papyrifera |
| 24 | L. angustifolia and J. Procera | C. martini, R. officinalis | B. papyrifera |
| 25 | L. angustifolia and J. Procera | C. martini and F. vulgare | B. papyrifera |
| 26 | L. angustifolia and J. Procera | C. martini and T. schimperi | B. papyrifera |
| 27 | L. angustifolia, C. nardus | C. martini and F. vulgare | B. papyrifera |
| 28 | L. angustifolia, C. nardus and J. Procera | C. martini, T. schimperi | B. papyrifera |
| 29 | L. angustifolia, C. nardus and J. Procera | C. martini, F. vulgare | B. papyrifera |
| 30 | L. angustifolia, J. Procera, C. nardus | C. martini, T. schimperi | B. papyrifera |
Table 1. Essential oil classification based on fragrance notes and perfume preparation.
The prepared perfume were poured into black bottle then it were placed in the dark area until hedonic test. Women panelists were selected and testing was performed using 21 panelists by asking each panelist to smell formulated perfume sample and fill preference for each formulation. The assessment criteria of perfume formulation test were shown in table below (Table 2) [6].
| Number | Criteria | Score |
|---|---|---|
| 1 | Like very much | 5 |
| 2 | Like moderately | 4 |
| 3 | Like slightly | 3 |
| 4 | Neither like nor dislike (Less fragrant) | 2 |
| 5 | Dislike strongly | 1 |
Table 2. Perfume fragrance level criteria for panelist evaluation.
Essential oil yield
The result of essential oil content of Lavandula angustifolia, Cymbopogon martini, Rosmarinus officinalis, Cymbopogon nardus, Boswellia Papyrifera, Juniperus procera and Thymus schimperi is given in Table 3. Based on the fresh leaves mass basis the essential oil content of Lavandula angustifolia was 1.3% by mass. Sarkic and Stappen, reported that Lavandula angustifolia essential oil is a clear, colorless to pale yellow liquid with a characteristic odor which is extracted by steam distillation from the flowering tops of Lavandula angustifolia. The investigation done by Kara and Baydar, who compared four cultivars of Lavandula angustifolia, showed that content of oil was oscillated from 0.35% to 2.0%. While Zheljazkov, et al. reported lower content of essential oil 0.71%-1.30% in the dried lavender flowers. Seidler, et al. reported essential oil content of (3%) from dry Lavandula angustifolia flowers [7].
The essential oil content of Cymbopogon martini leaves was obtained 0.82% by wet mass basis. Padalia, et al., reported that essential oil yield of Cymbopogon martini was found to vary from 1.0%-1.4% in leaves. Clear and intense yellow brownish, pleasant smell essential oil (0.91% yield) was obtained from steam distillation of fresh Rosmarinus officinalis. 1.1%(w/w) oil yield with hydro distillation was reported by Asressu and Tesema. Comparable essential oil yield (1.08% on fresh weight basis) was reported by Iram, et al.
Crushed and steam distilled Juniper procera berries oil yield was 2.3%by partially dry basis. The oil content of Cymbopogon nardus leaves was found to be 0.636% from fresh leaves. Lower essential oil yield (0.5%) was obtained from fresh leaves of Cymbopogon nardus (1000 g) with steam distillation apparatus for three hours. Jawonisi, reported 1.03% and maximum yield of (Cymbopogon nardus) which was 1.37% (w/w) was reported by Nour, et al., based on a dry basis and the specific gravity value obtained 0.8960 is less than 1, indicating that the oil is less dense than water. This is further established by the boiling point of 74°C indicating that the oil is volatile and therefore composed of light molecular weight components. From steam distillation of fresh Thymus schimperi leaves 1.17 (w/w)% transparent, yellow or reddish brown pleasant essential oil was obtained. Mean content of Thymus schimperi oil yield oscillated between 1.12% wet leaves and 2.99% on the weight of the airdried leaves was reported. The essential oil yield of sohxlet extraction of Boswellia papyrifera was 5%. According to Boswellia papyrifera resin contains about 5%-9% essential oil, 65%-85% alcoholsoluble resin and the remaining 21%-22% is water soluble gum (polysaccharidic fraction and polymeric substances) [8].
| Plant material | Obtained value (%) |
Literatures value (%) |
|---|---|---|
| Lavandula angustifolia | 1.3 | 0.35-2 |
| Cymbopogon martini | 1.04 | 1-1.4 |
| Rosmarinus officinalis | 0.91 | 1.1 |
| Juniperus procera | 2.3 | - |
| Cymbopogon nardus | 0.83 | 1.03 |
| Thymus schimperi | 1.17 | 1.12-2.99 |
| Boswellia papyrifera | 5 | 5-9 |
Table 3. Essential oil content of selected aromatic plants.
Chemical composition of extracted essential oil
Comparative chemical composition of Lavandula angustifolia oil: The Table 4 shows that the chemical compounds in steam distillation extracted Lavandula angustifolia essential oil are camphor (21.19%), 4-methyl-1-(1-methylethyl)-bicyclo (3.1.0) hex-2-ene (16.29%), endo-borneol (14.08%), β-pinene (9.22%) and (1R)-2, 6,6- trimethylbicyclo(3.1.1)hept-2-ene (7.07%). According to Seidler, et al., lavender flowers contain essential oil and its components: Linalyl acetate (40%), linalool (30%), limonene, u-ocymene,1,8-cineole, camphor, I-terpineol, borneol, but also phenolic acids (rosmarinic acid), ursolic acid, cumarins (umbelliferone, herniarin) flawonoids and sterols. Also Soodabeh Saeidnia, et al. who evaluated aroma profile of lavender cultivated in Teheran, reported that the main compounds of lavender oil was linalool (31.0%), linalyl acetate (18.2%) and lavadulyl acetate (10.7%) [9].
According to Stashenko, Linalool is found in the essential oils of over 200 plant species, belonging to different families. For example, linalool and its ester form, linalyl acetate 2, are the lavender oil main constituents. Cal and Krzyzaniak, reported that in perfumery, linalool is a commonly used fragrant ingredient being a component of many perfumes top notes and being found in 60%-90% of cosmetic products. Essential oil and extracts derived from lavender flower are commonly used as cosmetics, fragrance industry, perfumes and hygiene products (Table 4) [10].
| Compounds | RT (min) | Composition (%) |
|---|---|---|
| Camphor | 13.919 | 21.19 |
| 4-methyl-1-(1-methylethyl)-bicyclo (3.1.0) hex-2-ene | 9.221 | 16.29 |
| endo-borneol | 14.816 | 14.08 |
| 1,8-cineole | 9.485 | 10 |
| β-Pinene | 7.376 | 9.22 |
| (1R)-2, 6, 6-trimethylbicyclo[3.1.1]hept-2-end | 6.07 | 7.07 |
| β-Myrcene | 7.837 | 3.62 |
| a-Terpeneol | 15.861 | 3.48 |
| 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-naphthalene | 29.483 | 3.37 |
| 3,7-dimethyl-1,6-octadien-3-ol | 11.99 | 2.5 |
| β-Ocimene | 9.583 | 1.93 |
| β-Phellandrene | 7.273 | 1.82 |
| Linalool | 12.43 | 1.08 |
| a-Bisabolol | 35.961 | |
| Total components composition identified | 95.63 |
Table 4. Chemical composition of Lavandula angustifolia oil.
Comparative chemical composition of Cymbopogon martini oil:
The Table 5 shows that the compounds of Cymbopogon martini essential oil that is extracted by steam distillation process. GC-MS results show some components have high concentrations such as geraniol (40.89%); Tricyclo(2.2.1.0)(2,6)heptane, 1,3 Trimethyl (13.91%), β-Myrcene (9.34%),2,4,6,octatriene,2,6 dimethyl (8.20%), β- ocimene (5.93%) and a minor concentration of hexadecanoic acid, bisabolone, 11-octa decenoic acid ,methyl stearate, 4- undeccanone, citral , citronellol , caryophyllene [11]. From the GC-MS analysis C. martini it is found that large composition of geraniol, this is supported by Mohamed Yousif, it is reported that C. martini has geraniol (67%-85%) as the major component. Geraniol is naturally occurring terpenoid found in plants, is often used as a fragrance or ingredient in cosmetics and used as perfumery raw material for imparting rose like aroma in soaps and cosmetics products. C. martini is great prospects for producing quality essential oils and they have direct relevance to the perfumery industry with economic benefit to humankind. The ocimenes are monoterpenes found within a variety of plants and fruits. α-ocimene and the two β- ocimenes differ in the position of the isolated double bond [12].
| Compound | RT | Composition (%) |
|---|---|---|
| Geraniol | 11.3352 | 40.89 |
| Tricyclo(2.2.1.0)(2,6)heptane, 1,3 trimethyl | 12.5382 | 13.91 |
| β-Myrcene | 6.196 | 9.34 |
| 2,4,6,octatriene,2,6 dimethyl | 8.5281 | 8.2 |
| β-ocimene | 7.1391 | 5.93 |
| Hexadecanoic acid | 18.975 | 5.3 |
| Bisabolone | 17.8362 | 3.93 |
| 11-Octa decenoic acid | 22.0138 | 3.48 |
| Methyl stearate | 22.2712 | 3.18 |
| 4-Undeccanone | 11.182 | 2.3 |
| Citral | 11.7896 | 1.43 |
| Citronellol | 10.9065 | 1.15 |
| Caryophyllene | 12.636 | 0.96 |
| Total components composition identified | 96.52 |
Table 5. Chemical composition of Cymbopogon martini essential oil.
Comparative chemical composition of Rosmarinus officinalis oil: Table 6 shows that the chemical composition of Rosmarinus officinalis essential oil extracted by steam distillation process. From the GC-MS results the main chemical compounds in Rosmarinus officinalis are α-pinene (38%), eucalyptol (27.76%), caryophyllene (7.37%), bornanone (7.27%) and a minor components are β-pinene, D-limonene,endo-orneol,camphor,tricyclo(2.2.1.0(2,6))heptane,1,3,3- trimethyl, α-terpineol, bicyclo(3.1.1)hept-3-en-2-one, 4,6,6-trimethyl-, (1S), Humulene.
Sarkic and Stappen, reported that the main chemical compounds in rosemary essential oil are eucalyptol (19.4%) and α-pinene (14.7%). Camphor (9.5%), bornyl acetate (9.1%), camphene (6.9%), β-pinene (6.7%), β-myrcene (5.8%), limonene (5.2%) and borneol (5.0%) are also found in the oil. According to Porte, et al., the major constituents of the oil were camphor (26.0%), 1,8-cineole (22.1%), myrcene (12.4%) and α-pinene (11.5%). Iram Ayoob, reported that the major compounds of the oil identified were α-pinene (16.33%), 1,8-cineole (14.33%), camphor (22.01%), camphene (9.28%), β- pinene (5.97%), β-phellandrene (5.19%), bornyl acetate (4.59%), myrcene (4.31%), borneol (3.35%), terpinen-4-ol (1.11%), α-terpineol (1.03%), verbenone (1.39%), γ-terpinene (1.04%), linalool (1.16%) and β-caryophyllene (2.88%). The major constituents reported are mostly monoterpenes, like α-pinene, 1,8-cineole and camphor with variable amounts of camphene, myrcene, limonene, borneol, verbenone, bornyl acetate etc. Difference in the chemical composition of essential oil depends upon number of factors such as environmental conditions, location, elevation, harvesting period, storage conditions [13].
| Name | RT | Content (%) |
|---|---|---|
| α-Pinene | 5.0921 | 38 |
| Eucalyptol | 7.0507 | 27.76 |
| Caryophyllene | 12.6348 | 7.37 |
| Bornanone | 9.8598 | 7.27 |
| β-Pinene | 5.921 | 4.23 |
| D-Limonene | 6.7214 | 3.82 |
| endo-Borneol | 10.2057 | 3.46 |
| Camphor | 11.3342 | 2.31 |
| Tricyclo(2.2.1.0(2,6))heptane, 1,3,3-trimethyl- | 8.8186 | 1.79 |
| α.-Terpineol | 10.3652 | 1.57 |
| Bicyclo(3.1.1)hept-3-en-2-one, 4,6,6-trimethyl-, (1S)- | 11.3804 | 1.52 |
| Humulene | 13.1316 | 0.9 |
| Total components composition identified | 99.1 |
Table 6. Chemical composition of Rosmarinus officinalis oil.
Comparative composition of Juniperus procera berries essential oil: As shown on Table 7 the major chemical composition of Juniperus procera essential oil is α.-Pinene (85.68%) and Bicyclo(3.1.0)hexane, 4-methylene-1-(1-methylethyl) (13%). The leaf oil of J. procera is dominated by α-pinene (22.3%), 3-carene (18.7%), trans-totarol (8.9%) and abietadiene (7.8%) as well as moderate levels (2%-4%) of elemol, α-eudesmol, myrcene, β-phellandrene, β- pinene and terpinolene. Alpha-pinene is an organic compound of the polyphenolic group terpene. It is a component of many aromatic plants. Liorens-molina, Vacas and Sabater, reported that the berries essential oil composition shows α-pinene (55.7%-65.0%) and myrcene (16.6%-22.6%) were the main compounds in berries. According to Salamon, the juniper essential oil, in particular, is high in α-pinene, β-myrcene, β-caryophylene and terpinen-4-ol, which have very advantageous aroma therapeutic properties.
| Compound | RT | Composition (%) |
|---|---|---|
| α.-Pinene | 5.0974 | 85.68 |
| Bicyclo(3.1.0)hexane, 4-methylene-1-(1-methylethyl)- | 5.9382 | 13.03 |
| 1,3,8-p-menthatriene | 9.7879 | 1.28 |
| Total components composition identified | 99.99 |
Table 7. Chemical composition of Juniperus procera berries essential oil.
Comparative chemical composition of Cymbopogon nardus essential oil: Table 8 shows that the components of Cymbopogon nardus essential oil that is extracted by steam distillation process. GC/MS results show more than eleven components citronellal (38.21%), citronellol (23.16%), 3-carene (14.26%),2,6-octadiene, 2,6- dimethyl (6.75%), D-Limonene (4.93%), of which the most important ones are 7-octenal,3,7-dimethyl, citronellol and 3-carene. According to Wibowo, et al., major constituents of the essential oil were citronella (26.27%), δ-cadinene (6.97%), methyl Isoeugenol (5.87%), caryophyllene (5.87%), geranyl butyrate (5.6%), geranyl acetate (4.41%), citronellyl propionate (4.97%). They are present at high concentrations in the oil and are responsible for perfume formulation. These are the substances to create the special scent of Cymbopogon nardus and the applications are in the production technology of cosmetics and perfume. Citronellol is a fragrance ingredient used in decorative cosmetics, fine fragrances, shampoos, toilet soaps and other toiletries as well as in noncosmetic products such as household cleaners and detergents. Delta 3 carene is terpene carrying a sweet and earthy aroma with piney undertones. Also found in rosemary, basil, bell pepper, cedar and turpentine, delta 3 carene is used in cosmetics, perfumes and is widely considered a natural antihistamine.
The scent of citronella oil (Cymbopogon nardus) is known to blend well with all citrus essential oils, such as lemon and bergamot, as well as with cedar wood, clary sage, eucalyptus, geranium, lavender, peppermint, pine, rosemary, sandalwood and tea tree essential oils. Used cosmetically or topically in general, citronella essential oil can deodorize and refresh foul body odors by inhibiting the growth of odor-causing bacteria, which makes it an ideal ingredient in natural perfumes, deodorants, body sprays and bath blends.
| Compounds | RT | Composition (%) |
|---|---|---|
| Citronellal | 9.615 | 38.21 |
| Citronellol | 10.902 | 23.16 |
| δ-3-Carene | 11.316 | 14.26 |
| 2,6-octadiene, 2,6-dimethyl- | 12.074 | 6.75 |
| D-Limonene | 6.7257 | 4.93 |
| Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- | 13.765 | 3.60 |
| (+)-3-Carene | 12.533 | 2.60 |
| Guaia-1(10),11-diene | 15.101 | 2.21 |
| Cyclohexane,1-ethenyl-1-methyl-2,4-bis(1- methylethenyl)-, (1S-(1.alpha.,2.beta.,4.beta.))- |
12.1756 | 2.08 |
| Naphthalene, 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)- | 13.498 | 1.21 |
| beta.-copaene | 12.465 | 0.98 |
| Total components composition identified | 99.99 |
Table 8. Chemical composition of Cymbopogon nardus essential oil.
Comparative chemical composition of Thymus schimperi essential oil: Table 9 shows GC-MS result of the T. schimperi oil obtained from Ankober resulted in the identification of 9 compounds. The main chemical compounds found in T. schimperi were o-thymol (58.21%), γ-Terpinene (23.75%), Thymol (3.42%), 3- Octanol (5.3%), α-terpinene (3.2%) and pcymene (2.87%). According to Dagne and Bisrat, it was reported that oil obtained from Addis Ababa was rich in Carvacrol (66.2%) and γ-Terpinene (13.2%).
| Compounds | RT | Composition (%) |
|---|---|---|
| o-Thymol | 22.018 | 58.21 |
| γ-Terpinene | 11.060 | 23.75 |
| Thymol | 21.354 | 3.42 |
| 3-Octanol | 10.352 | 5.3 |
| α-Terpinene | 16.725 | 3.2 |
| p-Cymene | 9.704 | 2.87 |
| Endo-borneol | 15.563 | 0.90 |
| Caryophyllene | 26.349 | 0.91 |
| Total components composition identified | 98.2 |
Table 9. Chemical composition of Thymus schimperi oil.
Comparative chemical composition of Boswellia papyrifera essential oil: As showed on Table 10 hexane extracted essential oil composition of oleo gum resins of B. papyrifera were (1S,2R,5E,9E, 12R)-12-Isopropyl-1,5,9-trimethyl-15 oxabicyclo(10.2.1) pentadeca5,9-dien-2-ol (53.%) and 2-cyclohexen-1-one, 3-methyl-6-(1 methylethylidene) (46.95%). According to Bekana, et al., the essential oil of B. papyrifera is mainly characterized by the presence of octyl acetate (57.1%-65.7%) and n-octanol (3.4%-8.8%).
Because of its noteworthy scent and use as an important fixative in perfumes, soaps, creams, lotions and detergents, the perfume and cosmetic industry has considerable interest in the production of frankincense. Methanol extract of B. papyrifera resin collected from Humera area revealed components with retention time of 20.87, 24.83, 24.95 and 26.25 min which were identified as incensyl acetate, β-amyrenone, β-amyrin and α-amyrin, respectively.
| Compounds | RT | Composition (%) |
|---|---|---|
| (1S,2R,5E,9E,12R)-12-Isopropyl-1,5,9-trimethyl-15-oxabicyclo(10.2.1)pentadeca-5,9-dien-2-ol | 24.4437 | 53.0463 |
| 2-Cyclohexen-1-one,3-methyl-6-(1 methylethylidene)- | 23.9118 | 46.9537 |
| Total components composition identified | 99.95 |
Table 10. Chemical composition of Boswellia papyrifera essential oil.
Perfume formulation and Hedonic test
In this study, an effort was made to prepare perfumes using seven fragrance essential oil and alcohol as a solvent with different mixture proportion of the fragrance oil. Ethanol was used as a solvent for all perfume samples preparation. The prepared samples were prepared by mixing the extracted eight fragrant oils, thirty different perfume samples with 15 ml volume, at room temperature, at 35°C and at 45°C were analyzed.
A perfume is valued both for its appearance and its fragrance. Perfumes come in many colors, including clear, gold and brown. These colors are a consequence of the natural ingredients used to create the perfume. Since exposure to heat adversely affected each of these characteristics, hot temperatures can decrease a perfume’s value. Excess heat can alter the top notes of a perfume. Hot temperatures can cause a perfume’s color to darken or to become more cloudy and opaque.
According to Mandavgane, et al., alcohol base samples exhibited good perfumery properties. The prepared perfume samples were tested on human skin. After establishing the perfume formulations the prepared samples were presented about the products and an assessment was done based on each participant preferences of perfume aroma. The samples were given to a group of people of different age, gender and social status and feedback was collected by the prepared hedonic test about the aroma (Figure 1).
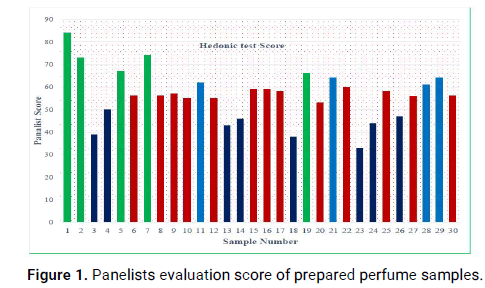
Figure 1: Panelists evaluation score of prepared perfume samples.
From the panelist hedonic test result, statistically the formulas with greater preference are formulation number 1,2,5,7 and 19. As showed in Figure 1, ingredients of sample number 1 were Lavandula angustifolia as top note; Cymbopogon martini as a middle note and Boswellia papyrifera as a base note. Ingredients of sample 2 were Cymbopogon nardus as top note; Rosmarinus officinalis as a middle note and Boswellia papyrifera as base note. The third sample was sample number 5 and the ingredients were Lavandula angustifolia and Juniperus procera as a top note, Cymbopogon martini and Foenculum vulgare as middle note and Boswellia papyrifera as a base note. This indicates that the aroma of the above fragrance essential oils are pleasant.
For this reason, preference is given to formula that contain Lavandula angustifolia and Cymbopogon nardus as top note; Cymbopogon martini and Rosmarinus officinalis as a middle note and Boswellia papyrifera as a base note.
Lower value of panelist response was given for sample number 23, 3 and 18. The ingredients of these samples were Juniperus procera, Cymbopogon nardus and Lavandula angustifolia as a base note; Thymus schimperi as a middle note and Boswellia Papyrifera as a base note. The samples which has lower response value contained Thymus schimperi essential oil and this indicates that the presence of this essential oil has negative effect on the scent of the perfume because thymus schimperi take over the other essential oil aromas and create strong spicy scent, that is not satisfied the panelist judgment.
Essential oils and their fragrance compounds are a very important part of perfume and cosmetic industry as they can serve as natural or natural-like chemical preservatives and at the same time, offer various benefits for skin and body. Additionally, these chemicals increase the value of cosmetic products due to their pleasant odor.
From this perfume formulation research, it is observed that the ancient technology of perfumery is pretty rich. By using seven fragrance essential oil and alcohol using the standard perfumes note percentage and different combination of the ingredient, thirty different perfume samples were prepared using eight raw materials. The technology is cheap and easy. This is a successful attempt to explore the possibility and feasibility of perfume manufacturing using plant leaves and flowers fragrance essential oil. The panelist gave a satisfactory response for perfume samples that contain Lavandula angustifolia and Cymbopogon nardus as top note; Cymbopogon martini and Rosmarinus officinalis as a middle note and Boswellia papyrifera as a base note.
We are grateful to express our deepest thank for Debre Berhan university research and community service directorate for funding the research. Our gratitude also goes to Dr. Minbale Gashu and Dr. Amare Ayalew and manuscript reviewers for their constructive comments and thoughtful suggestions on the manuscript.
We declare that we have no competing interests.
[Crossref] [Google Scholar] [PubMed]
Citation: Gisila T, et al. "Extraction of Essential Oil from Locally Available Aromatic Plants and Formulation of Natural Perfume". Nat Prod Chem Res, 2024, 12(1), 1-8.
Received: 10-Mar-2020, Manuscript No. NPCR-24-3593; Editor assigned: 13-Mar-2020, Pre QC No. NPCR-24-3593 (PQ); Reviewed: 27-Apr-2020, QC No. NPCR-24-3593; Revised: 03-Jun-2024, Manuscript No. NPCR-24-3593 (R); Published: 30-Jun-2024, DOI: 10.35248/2329-6836.24.12.1.1-8
Copyright: © 2023 Gisila T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.