Research Article - (2016) Volume 4, Issue 1
This study was carried out in methanol extracts of the dried leaves of Amaranthus spinosus were collected and used for phytochemicals, physiochemical and antibacterial analysis. By detecting the MIC (Minimum Inhibitory Concentration) and zone inhibition, the antibacterial activity was determined against different bacterial and fungal strains. The extract yields from the leaves was 6.97 methanol w/w. Phytochemical investigation of this plant determines that tannins 6.07%, Saponins 53%, alkaloids 13.14%, proteins 16.76% and glycosides 63.2% were rich in leaves. The extracts also contained appreciable levels of total phenolic contents 2.81 in GAE, g/100 g, total flavonoid contents 18.4 QE, g/100 and the free radical scavenging activity, showing IC50 (83.45 μg/mL) along with reducing power was calculated. The minimum inhibition concentration of extracts shows that 178 μg/mL. The physiochemical result obtained can be used for the identification of powdered drugs. The whole plant is used as a laxative. Traditionally it has been used as diuretic, antidiabetic, antipyretic, anti-snake venom, antileprotic, anti-gonorrheal, anti-inflammatory, and anthelmintic and immunomodulatory. The root paste of the plant is used to cure skin disease. A red pigment obtained from the plant is used for coloring foods and medicines. The results of this study suggest the possibility of using the methanol extract in treating the diseases caused by the tested organisms.
Keywords: Amaranthus spinosus; Plant; Physiochemical; Phytochemical; Leaves; Extract phytochemicals; DPPH; Lineolic acid; Reducing power
Amaranth is easy to grow, nutrient rich and underutilized pseudocereal that can play an important role in actions against hunger and malnutrition that occur due to low rainfall conditions [1]. Amaranth grows rapidly and has a high tolerance to arid conditions and poor soils where traditional cereals cannot be grown. According to Monica et al. Amaranth has been touted as a miracle grain, a super grain, and the grain of the future [2]. Amaranth was a staple in the diets of ancient Aztecs and Incas who believed it had supernatural powers and the grain was part of religious ceremonies and also used in the making of religious statues. It has been cultivated as a grain for 8,000 years, dating back to the Maya culture of South and Central America [3] and a grain yield of up to 8,000 kg/ha has been reported [4]. Because it is easy to digest, Amaranth is traditionally given to those who are recovering from an illness or a fasting period. In Mexico, grain Amaranth is popped and mixed with a sugar solution to make a confection called "alegria" (happiness). A traditional Mexican drink called "atole" is made from milled and roasted Amaranth seed [5]. In India, A. hypochondriacus L. is known as "rajgeera" (the King's grain) and is often popped to be used in confections called "laddoos" which are very similar to Mexican "alegria". In Nepal, Amaranth seeds are eaten as gruel called "sattoo" or milled into a flour to make chappatis [6]. Amaranth, a legacy of the Atecs, Mayas, and Incas, continues to be an under-exploited plant with a promising economic value due to the variety of uses it can have and the benefits it can provide to producers, processors, and consumers [7]. The Amaranth plant is also attractive since it adapts itself to a large number of environments, grows with vigor, produces large amounts of biomass, and resists drought, heat, and pests [8]. The Amaranth grains contain large amounts of dietary fiber, iron, calcium, lysine, methionine and cysteine, combined with a fine balance of amino acids, making them an excellent source of high quality, balanced protein, which is more complete than the protein found in most grains [2,9].
Amaranth grain is a pseudo-cereal and gluten-free used in breakfast cereals, pancakes, soup, breads, cookies, gluten-free foods, extruded snacks and as an ingredient in confections [1]. South Americans parch or cook it for a gruel or porridge, or mill it to produce light-colored flour. As a snack, the grain is popped and tastes like a nutty-flavored popcorn. It can also be mixed with honey [10]. Ljubica et al. have reported that Amaranth flour can be used to partially replace regular corn flour for extruded snack manufacturing [11]. Xaene et al. also reported that mixture of instant whole Amaranth and rice can be used to produce extruded flours to be used in formulations of beverages [12]. According to Rosa et al. extruded snacks can be manufactured from defatted Amaranth flours [13].
The pharmacological properties of Amaranth products are considered of vital importance Rosa et al. [13]. For reducing tissue swelling the leaves are well thought-out to be constructive, and they have a cleansing effect too. The plant has also been used curatively for diarrhea, dysentery, excessive menstrual flow, ulcers and intestinal hemorrhaging. For the treatment of intestinal bleeding, excessive menstruation, diarrhea and other related problems, a tea made from its leaves are used [14]. The aim of the present study was to evaluate physiochemical, phytochemicals, and antioxidant and to detect the presence of natural therapeutic agents, especially those related to control the microbes that cause diseases in human beings from Amaranthus spinosus leaf extracts with absolute and 80% methanol solvent system.
Collection of plant material
Matured leaves of Amaranthus spinosus L. were collected from the fields of Mekelle, which is a city in the Tigray province, Ethiopia. These plants were identified by the Department of biotechnology, University of Mekelle, Ethiopia. The matured leaves from the plant were selected because there is a maximum metabolism in fully matured leaves as compared to young leaves. Specimens were dried at room temperature and stored in polyethylene bags at 4°C.
Preparation of plant extract
Methanol extraction of the plants material was carried out by suspending 100 grams of the powders of Amaranthus in 1000 ml of absolute methanol and 80% methanol (80:20, methanol: water, v/v). The extraction was allowed to stand for 72 hours at 37°C. The extracts were filtered first through cotton wool, then through Whatman filter paper No.1 (125 mm) and were dried using a rotary evaporator. They were transferred into sterile bottles and kept in refrigerator until used.
Phytochemical analysis
Phytochemical screening for the presence of Tannins, alkaloids, glycosides, flavonoids, and phenolic was performed using standard procedures [15].
Qualitative analysis of phytochemicals alkaloids: The extracts were evaporated to dryness and the residues were heated on a boiling water bath with 2% Hydrochloric acid, cooled, filtered and treated with the Mayer’s reagent. The sample was then observed for the presence of yellow precipitation or turbidity [15].
Flavonoids: 1.5 ml of 50% methanol was added to 4 ml of the extracts. After warming add magnesium fillings followed by a few drops of concentrated hydrochloric acid. A pink or red color indicates the presence of flavonoid [15].
Tannins: A portion of the extract was diluted with distilled water in a ratio of 1:4 and a few drops of 10% ferric chloride solution were added. A blue or green color indicates the presence of tannins [16].
Saponins: A small quantity of the methanol extract was boiled. The mixture was filtered and 2.5 ml of the filtrate was added to 10 ml of the distilled water in a test tube and shake well for about 30 seconds and observed for frothing [15].
Glycosides: In a methanol extract Fehling’s reagent was added and boiled for 2 minutes. A brick red coloration indicates the presence of glycosides.
Quantitative analysis, estimation of alkaloids contents: The alkaloid content of extract was determined [17]. About 5.0 g of each sample was weighed into a 250 ml beaker, and a 200 ml of 10% acetic acid in ethanol was added, and allowed to stand for 4 hrs. This was filtered using Whatman No. 42 filtered paper, concentrated in water bath to one fourth (50 ml) of the original volume, then, concentrated NH4OH was added drop wise to each alkaloid extract until the precipitate was complete. The suspension was allowed to settle and the precipitate was collected, washed with NH4OH and then filtered. The residue was dried and weighed. The percentage alkaloid was then calculated.
Estimation of tannin content: 500 mg of plant sample was weighed and transferred to a 50 ml flask. Then added 50 ml of distilled water and stirred for 1 h. The sample was filtered into a 50 ml volumetric flask and the volume was made up to the mark. 5 ml of the filtered sample was pipette into test tube and then mixed with 2 ml of 0.1 M ferric chloride. The absorbance was measured using spectrophotometer at 395 nm wavelength within 10 min [18].
Estimation of saponins content: Saponins content was determined by the reported method [19]. About 2.0 g of each extract was mixed with 100 ml of 20% ethanol, and were incubated in a water bath at 55°C for 4 hrs, with stirring. The mixture was filtered and the extract was re-extracted with 200 ml of 20% ethanol. The combined extract was concentrated to 40 ml in a water bath at 90°C. The concentrate was then transferred into a 20 ml separator funnel and 20 ml di-ethyl ether was added, and shaken vigorously. The aqueous layer was recovered while the outer layer was dis-carded, the purification process was heated and 60 ml of n-butanol added. The combined n-butanol extracts were washed twice with 10 ml of 5% aqueous NaCl, and the remaining solution was heated in a water bath. After evaporation, the samples were dried in the oven to a constant weight, and the percentage Saponins content was calculated.
Estimation of glycosides: The glycosides content of the extracts was determined by dissolving 5.0 g of the extracts in 50 ml of 50% H2SO4 in test tubes. The mixture was heated in boiling water for 15 minutes, and 5 ml of Fehling solution added, and the mixture boiled. A red precipitate in each extract tested, indicated the presence of glycosides. The percentage glycoside was calculated [15].
Antioxidant activity
Total phenolic contents (TPC) and total flavonoid contents (TFC): Total phenolic contents (TPC) were determined using the Folin-Ciocalteu reagent method and Gallic acid was used as Gallic acid Equivalent (GAE). The total flavonoid contents (TFC) in the leaf extracts was determined following the modified procedure and Quericitin was used as standard as Quericitin Equivalent (QE).
DPPH radical scavenging assay: The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical asses was carried o
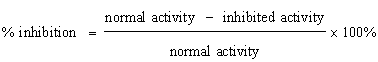
A blank the absorbance of the control reaction (containing all reagents except the test sample), and A sample is the absorbance of test samples. IC50 values, which represents the concentration of Amaranthus spinosus that caused 50% inhibition, were calculated from the plot of percentage against concentration.
Determination of antioxidant activity in linoleic acid system: The antioxidant activity of the Amaranthus spinosus extracts materials were also determined by measuring the oxidation of linoleic acid 5 mg of Amaranthus spinosus extracts were added separately to a solution of linoleic acid (0.13 mL), 99.8% ethanol (10 mL) and 10 mL of 0.2 M Sodium Phosphate buffer (pH=7). The mixture was made up to 25 mL with distilled water and incubated at 40°C up to 360 hours. Extent of oxidation was measured by peroxide value applying Thiocyanate method. Briefly, 10 mL of ethanol (75% v/v), 0.2 mL of an aqueous solution of Ammonium Thiocyanate (30% w/v), 0.2 mL of sample solution and 0.2 mL of ferrous chloride (FeCl2) solution (20 mM in 3.5% HC1; v/v) added sequentially. After 3 min of stirring, the absorption was measured at 500 nm using a spectrophotometer (U-2001, Hitachi Instruments Inc. and Tokyo, Japan). A negative control contained all reagents with exception of extracts. Synthetic antioxidants Butylated Hydroxytoluene (BHT) (also we can use ascorbic acid) was used as positive control. The maximum per oxidation level was observed at 360 h (15 days) in the sample that possesses no antioxidant component percent inhibition of linoleic acid oxidation was calculated with the following equation:
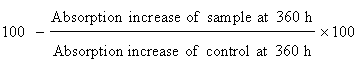
Determination of reducing power: The reducing power of the Amaranthus spinosus extracts were determined according to the spectrophotometric method [20] concentrated extract (0-10.0 mg) was mixed with sodium phosphate buffer (5.0 mL, 0.2 M, pH 6.6) and potassium ferricyanide (5.0 mL, 1.0%); the mixture was incubated at 50°C for 20 min. Then 5 mL of 10% Trichloro Acetic Acid was added and the mixture centrifuged at 980 g for 10 min at 5°C in a refrigerated centrifuge (CHM-17; Kokusan Denki, Tokyo, Japan). The up-per layer of the solution (5.0 mL) was decanted and diluted with 5.0 mL of distilled water and ferric chloride (1.0 mL, 0.1%), and absorbance read at 700 nm using a spectrophotometer (U-2001, Hitachi Instruments Inc., Tokyo, Japan). All samples were analyzed thrice and the results averaged.
Antimicrobial activity
Microbial strains: Staphylococcus aureus and Escherichia coli, together with two pathogenic fungi (Fussarium solani and Rhizopus oligosporus) were used as microbial strains. The organisms were bring from Ayder Referral hospital. These bacterial and fungal strains were cultured at 37°C and 28°C overnight in an incubator (Memmert Germany).
Disc diffusion method: The antimicrobial activity of the prepared extracts was determined by using disc diffusion method [21]. The inoculated extracts were then examined for inhibition zones (in mm) by zone reader, which indicates antimicrobial activity. The discs (6 mm in diameter) were impregnated with 20 μg/m, sample extracts (20 μg/ disc) and placed on inoculated agar. Rifampicin (20 μg/disc) (Oxiod) and Fluconazol (20 μg/disc) (Oxiod) were used as positive reference for bacteria and fungi, respectively [21].
Determination of minimum inhibitory concentration (MIC): The medium used in this assay was Isosensitest and standardized bacterial colony numbers was taken, adopting 5 × 105 CFU/mL final concentration of strains of bacterial species. After preparing microbial culture and resazurin solution glass plates were prepared. These were all prepared under aseptic conditions. A 96 well plate was sterilized and labeled. 100 μL of test material in 10% (v/v) DMSO/sterile water (10 mg/ mL for crude ex-tracts) was pipetted into the first row of the plate. In the rest of the wells 50 μL of nutrient broth was added and serial dilutions were made by adding 50 μL of the test sample in serially descending concentrations. In each well 10 μL of the resazurin indicator solution was added. At the end 10 μL of bacterial suspension (5 × 106 CFU/ mL) was added to each well to get a concentration of 5 × 105 CFU/mL. To avoid bacteria to become dehydrated each plate was wrapped loosely with cling film. Every plate had a set of control column having broadspectrum antibiotic as positive control (Ampicillin for bacteria: Benzyl penicillin for fungi). That column has all solutions except the test compound, and another column with all solutions with the exception of the bacterial solution adding 10 L of nutrient broth instead. The plates were prepared in triplicate, and placed in an incubator set at 37°C for 18-24 h. The color change was then assessed visually. Any color changes from purple to pink or colorless were recorded as positive. The lowest concentration at which color change occurred was taken as the MIC value. The average of three values was calculated and that was the MIC for the test material and microbial strain and the physiochemical properties of the plant can be determined using standard methods of AOAC (20).
Results
The study was carried out on the plant samples revealed the presence of medicinally active constituents. The phytochemical characters of Amaranthus spinosus were investigated and summarized in Tables 1 and 2. Alkaloids, tannins, saponins and glycosides were present. Quantitative estimation of the percentage crude chemical constituents in Amaranthus spinosus was studied and summarized in Table 2. Table 3 contains the percentage yield and antioxidant activity of Amaranthus spinosus leaf extracts. The highest DPPH activity, TP and TF content was found in 100% methanol leaf extracts as shown in Table 3.
| Phytochemicalconstituent | Amaranthusspinosus Leaves |
|---|---|
| Tannins | + |
| Saponins | + |
| Flavonoids | + |
| Cardiac glycosides | + |
+: Presence of the phytochemical constituent; −: Absence of the phytochemical constituent
Table 1: Qualitative analysis of phytochemical constituents of leaf extracts of Amaranthus spinosus.
| Part of plant used | Alkaloids | Tannins | Saponins | Glycosides | Proteins |
|---|---|---|---|---|---|
| Leaves | 13.14 ± 0.86 | 6.07 ± 0.93 | 53.0 ± 0.50 | 63.2 ± 0.80 | 16.76 ± 1.02 |
Table 2: Quantitative analysis for phytochemical constituents of leaf extracts of Amaranths spinosus.
| Part of plant used | Methanol extract | % age yield(g/100 g of DM) | TP contents* | TP contents** | DPPH assay (μg/mL)IC50 |
|---|---|---|---|---|---|
| Leaves | 100% | 5.5 ± 0.13 | 1.4 ± 0.5 | 2.78 ± 0. 20 | 14.27 ± 2.2 |
| 80% | 6.1 ± 0.14 | 2.81 ± 0.2 | 18.4 ± 0. 30 | 83.45 ± 3.87 |
Values are mean ± SD of samples analyzed individually in triplicate. *Total phenolic contents in Gallic acid equivalent. **Total flavonoid contents in Quericitin equivalent.
Table 3: Percentage yield and antioxidant activity of Amaranths spinosus leaf extracts.
The higher percentage inhibition of linoleic acid oxidation by the extract compared with the reference drug, BHT, suggests a marked and higher antioxidant activity Table 4. Furthermore, the percentage inhibition of linoleic acid oxidation (67.57%) obtained for the Amaranthus spinosus in this study is similar to that (68.41%) reported by Ref. [22]. At physiological pH (7.4), ferrous ions (Fe2+), in the presence of oxygen and phosphate ions (PO24), exist only transiently before being auto-oxidized to ferric ion (Fe3+). During this process, an electron is transferred from iron to oxygen to form a superoxide radical anion and hydroperoxyl radical (HO2•) by Fenton reaction.
| Inhibition ofper oxidation | Extracts | |
|---|---|---|
| % | 100% | 80% |
| 59.2 ± 1.7c | 67.4 ± 2.1b | |
Values (mean ± SD) are of three samples of Amaranths spinosus extract, analyzed individually in triplicate. Means followed by different superscript letter in the same row present significant difference (p<0.05).
Table 4: Percent inhibition of linoleic acid per oxidation of leaf of Amaranths spinosus extracts.
The concentration dependent, high reducing power of the aqueous extract of Amaranthus spinosus leaves suggests that the extract possessed the ability to be effective, under physiological conditions, in reducing the transition state of iron and consequently, the rate at which super oxide and hydroperoxyl radicals are generated from the metal Table 5. A strong relationship between the total phenolic content and reducing activity in fruits and vegetables has been reported [20]. Therefore, the reducing power of the extract may be attributed to its phenolic content.
| Concentrationmg/ml | Leaves | BHT | |
|---|---|---|---|
| 100% | 80% | ||
| 2 | 0.06 ± 0.01 | 0.08 ± 0.01 | 0.73 ± 0.04 |
| 4 | 0.39 ± 0.01 | 0.26 ± 0.08 | 0.95 ± 0.05 |
| 6 | 0.65 ± 0.04 | 0.58 ± 0.10 | 1.14 ±0.06 |
| 8 | 0.84 ± 0.05 | 1.16 ± 0.11 | 1.54 ±0.08 |
| 10 | 1.27 ± 0.06 | 1.60 ± 0.13 | 1.80 ±0.09 |
Values (mean ± SD) are of three samples of Amaranths Spinosus extract, analyzed individually in triplicate. Means present significant difference (p<0.05)
Table 5: Reducing power of different Amaranths spinosus leaf extracts.
Antimicrobial activity
The leaves of Amaranthus spinosus extracts showed consider antimicrobial activities in the disc diffusion assay. The quantitative estimation of antimicrobial activity of Amaranthus spinosus leaf extracts against food-borne and pathogenic microorganisms are shown in Tables 6 and 7. In leaf two solvent systems show different result as: 100% methanol leaf extract (25 mm) >80% methanol leaf extract (23 mm). The trend for antifungal activity was same to that of antibacterial activity except that the efficacy towards fungal strain was not much effective as for bacterial strains. The trend for antifungal activity was 100% methanol leaf >80% methanol. The current results support the earlier findings which demonstrate the presence of antimicrobial activity in leaves of Amarathaceae [23,24] (Tables 8 and 9).
| Part ofplant used | Methanolextract | Zones of growth inhibition mm | |||
|---|---|---|---|---|---|
| S. aureus | E. coli | F. solani | R. oligosporus | ||
| Leaves | 100% | 24 | 16 | 17 | 9.0 |
| 80% | 23 | 12 | 15 | 8.0 | |
| Control* | 100 % | 26 | 17 | 18 | 16 |
*Control used was Ampicillin (S. aureus, E. coli) and Benzyl penicillin (F. solani, R. oligosporus)
Table 6: Antimicrobial activities of Amaranths spinosus methanol leaf extract (100% and 80%).
| Part ofplant used | Methanol | Minimum Inhibitory Concentration (MIC) | |||
|---|---|---|---|---|---|
| extract | S. aureus | E. coli | F. solani | R. oligosporus | |
| Leaves | 100% | 179ab ± 1.28 | 398a ± 1.26 | 436 ± 1.46 | 302c ± 1.36 |
| 80% | 182ab ± 1.68 | 603b ± 2.36 | 491b ± 1.76 | 352b ± 2.42 | |
| Control Methanol | 141a ± 1.31 | 381a ± 2.39 | 391a ± 2.48 | 436a ± 2.17 | |
Extractive values obtained from Amaranthus Spinosus leaves
Table 7: Minimum inhibitory concentration of Amaranths spinosus leaf methanol extract against the selected bacterial and fungal strains.
| Extracts | Color | Consistency | % yield(w/w) |
|---|---|---|---|
| Petroleum ether | Dark green | Sticky | 2.56% |
| Chloroform | Blackish green | Sticky | 1.72% |
| Acetone | Brown | Semisolid | 0.98% |
| Methanol | Dark green | Semisolid | 6.97% |
Table 8: Data showing color, consistency and yields of different extracts of powdered leaf of Amaranths spinosus.
| Physical parameters | Percentage (w/w) |
|---|---|
| Total ash | 06.8% |
| Acid insoluble ash | 01.20% |
| Water soluble ash | 01.60% |
| Alcohol Soluble Extractive Value | 06.65% |
| Water Soluble Extractive value | 12.25% |
| Moisture content | 08.90% |
Table 9: Data showing physiochemical parameters of different extracts of powdered leaf of Amaranthus spinosus.
Evaluation of all the data demonstrates that Amaranthus spinosus leaves contain phytoconstituents like fixed oils and fats, carbohydrates, glycosides, gum, mucilage, phenolic compounds, protein amino acids, tannins and saponins which may be responsible for various pharmacological actions. Physiochemical evaluation is an important parameter to identify the drug and to establish its quality and purity. Ash values are used to determine the presence of impurities like inorganic salts, carbonates, phosphates, silicates and silica. Adhering dirt and sand is determined by acid insoluble ash and the inorganic elements present in the drug is determined in water soluble ash value. The moisture content of the drug should be at a minimal level to avoid microbial growth during storage.
This report was the first in the country Ethiopia. From the research project, it was concluded that the edible plant species Amaranthus spinosus from underutilized plant family had a rich amount of valuable ingredients that are beneficial for health the physiochemical and phytochemical parameters and this can be useful to identify the drug and to establish its quality and purity.