Research Article - (2021) Volume 12, Issue 5
The aim of the study is to evaluate the antidiabetic activity of aqueous peel extracts of selected fruits viz. Annona squamosa, Cucumis melo, Actinidia deliciosa, Malus pumila. Male wistar albino rats (180-200 g) divided into 12 groups including 1 control group. After induction of diabetes using alloxan, they were treated with selected extracts and polyherbal mixture. At the end of 28th day the animals were sacrificed; blood and pancreatic tissue samples were collected and analysed. All the selected fruit peel extracts and their polyherbal mixture shown significant reduction in the levels of blood glucose and serum lipids such as triglycerides, total cholesterol, LDL, VLDL but increase in HDL, insulin levels. The better action at lowest dose was shown by polyherbal mixture and Malus pumila extract followed by other peel extracts. Histopathological analysis of pancreatic tissue was in concurrence with the biochemical results These findings support that the selected fruit peel extracts and poly herbal mixture showed potent therapeutic effect on diabetes mellitus and can be a potent antidiabetic agent due to their ameliorating effects on damaged pancreas.
Fruit peels; Antidiabetic activity; Hypolipidemic activity; Polyherbal mixture
Diabetes mellitus (DM) is one among a major chronic endocrine disorder associated with insufficient insulin production or its action, leading to generation of free radicals [1]. Impaired metabolism of glucose occurs due to insulin deficiency and causes hyperglycemia. Simultaneous generation of free radicals leads to oxidative stress that is associated with increased lipid peroxidation and secondary complications of DM. The incidence of diabetes is on surge and it is been a major cause for deterioration of human health after the two major killers i.e, cancer and cerebrovascular diseases [2].
As per the survey of International Diabetes Federation around 415 milloin individuals had DM in 2015 and this number is anticipated to rise to 642 million by 2040. Around 75% of subjects with DM board in low and middle income countries (LMICS). In monetary terms the worldwide burden of DM is gigantic with an estimated annual expenditure of 673 billion $ in 2015 that entrenched 12% of global health expenditure for that year [3]. Where as in urban areas of LMICs, diabetes is well recognize as a public health priority; recent prevalence information recommend that diabetes is increasing downside among rural populations likewise [4]. India is home to 69.1 million individuals with DM and is estimated to possess the second highest number of cases of DM worldwide after China in 2015. The complications that are explicit to diabetes include retinopathy, nephropathy, and neuropathy. Patients with all types of diabetes of adequate term, including insulin-dependent diabetes mellitus (IDDM) and non-insulin-dependent diabetes mellitus (NIDDM), are powerless against these complexities, which cause genuine dismalness [5].
The normal treatment of diabetes is oral hypoglycemic medications and life style management. However the hypoglycemic medications are just ready to control blood glucose level rather than restoring the illness. Finding the likely helpful treatment for diabetes stays a test for a long time [6].
Evidence has gathered showing that the production of reactive oxygen species (oxidative stress) may assume a significant job in the etiology of diabetic complications. This theory is upheld by proof that numerous biochemical pathways carefully connected with hyperglycemia (glucose autoxidation, polyol pathway, prostanoid combination, protein glycation) can build the creation of free radicals [7].
Even though a plethora of antidiabetic drugs are available in market, the search for safer and economic alternatives is still high. In this way a huge scope research is conducted on medicinal plants with antioxidant activity as an alternative to the current synthetic treatments for diabetes.
In recent years there is an increasing interest in the antioxidant activity of phytochemicals present in our dietary foods viz., fruits and vegetables. It is a proven fact that most of these phytochemicals with antioxidant activity are concentrated more in the outer parts such as peel.
Most of the peels of fruits and vegetables are thrown as wastes. This could only burden the environment. Now days the scenario has been changing and researchers are recovering many valuable bioactive molecules from the fruit and vegetable peels and proving their beneficial health attributes ranging from antioxidant, anti inflammatory to cancer, antiviral and cardio protective activities etc [8]. Many studies confirmed that in most fruits, the phenolic content in peels is much higher than in pulp [9].
Malus pumila, belonging to the family- Rosaceae (Apple) is a notable organic product, famous everywhere throughout the world because of its health benefits. The plant, for the most part beginning from focal Asia, has spread everywhere throughout the world. The entire organic product is eatable including the external skin. Apple contains high measure of nutrients like nutrient C and nutrient B12, minerals like calcium and phosphorous, and a rich wellspring of starch [10]. Canned apple and apple sauce manufacture are the sources of apple peel wastes. Studies have found that the peels of Malus pumila are rich in polyphenols and posses antioxidant and antiproliferative activity [11]. Annona squamosa is a medium-sized tree which belongs to the family Annonacea. Different parts of Annona squamosa, for example, fruits, seeds, leaves and barks have been utilized to treat numerous illnesses [12]. Investigations on the peels of the Annona squamosa (custard apple) revealed antimicrobial and antioxidant activities [13]. Actinidia deliciosa belonging to the family Actinidiacea is intensely cultivated all over the world. The fruit of Actinidia deliciosa has been acclaimed for its native and medicinal values. It contains several phytoconstituents belonging to category of triterpenoids, flavonoids, phenylpropanoids, quinones and steroids. The roots of Actinidia deliciosa has been used as a traditional drug in China for a long time and are reported as Chinese folk remedy for various diseases, such as hepatitis, pyorrhea, gingivitis, edema, rheumatoid arthritis, and also various forms of cancer. Kiwi fruit has been used as mild laxative and a rich source of Vitamins. The fruits, stems and roots are diuretic, febrifuge and sedative. The seeds are used as natural blood thinner [14]. There are scientific evidences on antioxidant, antibacterial and anticancer activity of Actinidia deliciosa (kiwi) fruit peels [15]. Cucumis melo Linn belongs to the family Cucurbitaceae. Main parts used are pulp, root, seeds and seed oil. It is having diuretic, emmenagogue, cooling, demulcent, aphrodisiac, galactagogue and astringent properties. Fruit has been used for several centuries to treat kidney disorders such as kidney and bladder stones, painful and burning micturition, ulcers in the urinary tract, suppression of urine and to treat cough, bilious diseases, hot inflammation of the liver, liver and bile obstruction, eczema, etc [16]. Studies revealed that the skin of Cucumis melo (musk melon) showed good antioxidant activity [17]. Hence the objective of this research work is to find the therapeutic benefits of four different fruit peels and their polyherbal mixture (PHF).
Preparation of peel extracts and PHF
Fresh fruits were purchased from local market and they are washed under running tap water and their peels were separated. They were shade dried separately over a month. The dried peels were powdered using a mechanical grinder. Each of the samples was approximately weighed and subjected to extraction by cold maceration with water (80 parts and 20 parts ethanol) for 72 hrs. After 72 hrs the sample were filtered and concentrated. The obtained aqueous extracts were powdered and stored for further use. Poly herbal mixture was prepared by taking equal quantities of the selected fruit peel extracts
APAS: Aqueous peel extract of Annona squmosa
APAD: Aqueous peel extract of Actinidia deliciosa
APCM: Aqueous peel extract of Cucumis melo
APMP: Aqueous peel extract of Malus pumila
PHF: Poly Herbal Mixture
Preliminary phytochemical analysis
The aqueous peel extracts were tested for various phytochemicals using the standard preliminary phytochemical screening methods.
Experimental animals
Albino Wistar rats (180-200 g) of male were used in the present study. Animals were obtained from the Mahaveer laboratories, Hyderabad, Andhra Pradesh, India. The animals were housed under standard environmental conditions (23 ± 1℃) with relative humidity of 50 ± 10% and maintain 12:12 dark and light cycle, maintained with free access to water and ad libitum standard laboratory diet (70% carbohydrates, 25% proteins, 5% lipids (Hindustan liver Bangalore). After randomization before the experiment, the rats were acclimatized for a period of two weeks. The animal housing, handling and all the experimental procedures were carried out in strict compliance with the Institutional Animal Ethics Committee regulations (Reg. No. 516/01/A/CPSCEA).
Experimental protocol
Male Wistar albino rats (180-220 g) were fasted overnight (12– 14 h) and their weight and fasting blood glucose level recorded with a glucometer and then made diabetic by a single intraperitoneal injection (a volume of 1 mL/kg) of freshly prepared alloxan monohydrate solution (20 mg/kg body weight). Alloxan was prepared by weighing according to individual animal weight and solubilized with 0.5 mL sodium citrate at pH 4.5 before injection. Food and water were presented to the animals 30 min. after administration of alloxan [18,19].
After induction of diabetes, the animals showing blood glucose levels above 350 mg/dL were selected for the study. The selected animals were grouped basing on their body weight. The animals were administered with standard gliclazide and selected peel extracts orally for a period of 14 days. Various parameters were estimated at the end of 14 days study period in all the animals. On the 14th day, blood samples were collected from all animals by puncturing retro-orbital plexus under mild ether anaesthesia, later animals were sacrificed and liver tissues were collected (Figure 1).

Figure 1: Effect of selected peel extracts and polyherbal mixture on blood glucose levels in Alloxan induced diabetic rats.
The rats were divided into following groups, each group consisting of six rats.
Group 1: Normal control
Group 2: Disease control (Alloxan)
Group 3: Standard (Gliclazide) (1mg/kg) p.o.
Group 4: Alloxan +Received aqueous peel extract of Annona squamosa (200 mg/kg)
Group 5: Alloxan +Received aqueous peel extract of Annona squamosa (400 mg/kg)
Group 6: Alloxan +Received aqueous peel extract of Actinidia deliciosa (200 mg/kg)
Group 7: Alloxan +Received aqueous peel extract of Actinidia deliciosa (400 mg/kg)
Group 8: Alloxan +Received aqueous peel extract of Cucumis melo (200 mg/kg)
Group 9: Alloxan +Received aqueous peel extract of Cucumis melo (400 mg/kg)
Group 10: Alloxan +Received aqueous peel extract of Malus pumila (200 mg/kg)
Group 11: Alloxan +Received aqueous peel extract of Malus pumila (400 mg/kg)
Group 12: Alloxan + Polyherbal formulation (PHF) (200 mg/kg)
Assessment of biochemical parameters
The blood samples were centrifuged at 3000 rpm (micro centrifuge) for 10 min to separate serum. The serum samples thus collected were used for the estimation of blood glucose levels, glycosylated hemoglobin levels (HbA1c), insulin, serum lipid profile like triglycerides, total cholesterol, high density lipoproteins (HDL), low density lipoproteins (LDL) and very low density lipoproteins (VLDL) were estimated at the end of 28 days study period in all the animals. All these methods were performed by following the standard methods of international Federation of clinical chemistry (IFCC). All the parameters were estimated using standard diagnostic kits.
Histopathological studies
Pancreas was carefully excised and washed in ice cold normal saline and pressed between filter paper pads and a portion of liver (one animal of each group) was preserved in 10% neutral formalin for histopathology studies.
Collected pancreatic tissues were processed and embedded in paraffin wax. Sections of 5-6 μm in thickness was cut and stained in hematoxylin and eosin dye, were observed microscopically for histopathological changes [20].
Statistical analysis
The data of the biochemical parameters were expressed as mean ± SEM. Results were analyzed statistically using one way analysis of variance (ANOVA) followed by DUNNETT’s multiple comparison test. The minimum level of significance was fixed at p<0.05.
The aqueous peel extracts of the selected fruits Annona squamosa, Actinidia deliciosa, Cucumis melo, Malus pumila were evaluated for antidiabetic activity in alloxan induced diabetic rats. The selected fruit peel extracts showed significant reduction in total blood glucose, glycosolated heamoglobin and total cholesterol levels and showed increased levels of insulin, triglycerides and successfully altered the lipid levels.
Preliminary phytochemical analysis
The aqueous peel fraction of Annona squamosa showed presence of alkaloids, phenolics compounds, saponins, triterpenes, carbohydrates and tannins. The aqueous fraction of Actinidia deliciosa showed presence of phenolics, triterpenes and steroids. The Cucumis melo aqueous fraction showed presence of flavanoids, saponins, carbohydrates, steroids and tannins. Malus pumila were tested for phytochemical screening resulted that the aqueous fraction showed presence of glycosides, flavanoids, terpenoids and steroids as shown in Table 1.
| Phytoconsti tuents | Annona squamosa | Actinidia deliciosa | Cucumis melo | Malus pumila |
|---|---|---|---|---|
| Alkaloids | + | - | - | - |
| Glycosides | _ | - | - | + |
| Phenolics | + | + | + | + |
| Carbohydrat es | + | - | + | - |
| Amino acids | _ | - | - | - |
| Saponins | _ | - | + | - |
| Tannins | + | - | + | - |
Table 1: Preliminary phytochemical screening of aqueous peel extracts of selected fruit peels
Effect of selected peel extracts on blood glucose, serum insulin and glycosylated hemoglobin levels: The effect of selected fruit peel extracts and PHF on blood glucose, glycosylated hemoglobin and serum insulin levels were given in Figures 2 and 3. Blood glucose and glycosylated hemoglobin levels were significantly elevated in Group 2 in comparison to Group 1. In contrast, glycosylated heamoglobin and blood glucose levels were reduced significantly (P<0.05) towards the normal levels in groups 3-12. The serum insulin of level in disease control rats is significantly low than that of control group (normal group). But the serum insulin levels were increased in groups 3-12 when compared to disease control (Group 2).
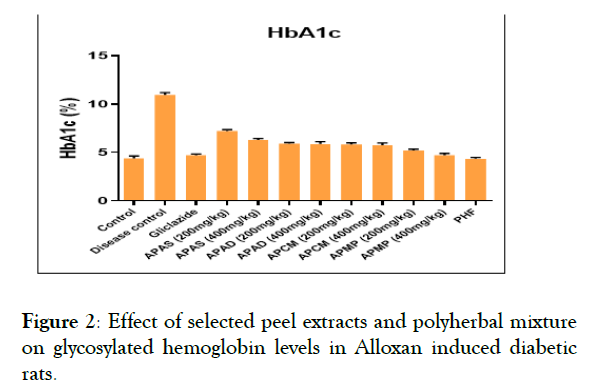
Figure 2: Effect of selected peel extracts and polyherbal mixture on glycosylated hemoglobin levels in Alloxan induced diabetic rats.
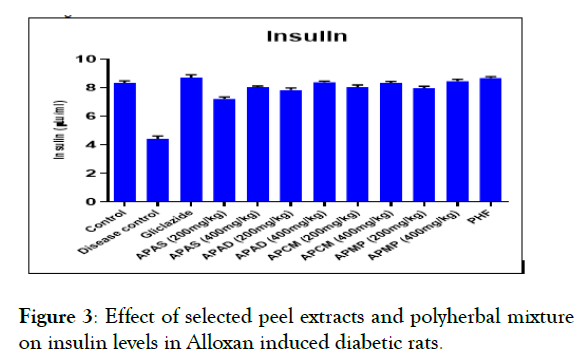
Figure 3: Effect of selected peel extracts and polyherbal mixture on insulin levels in Alloxan induced diabetic rats.
Effect of selected peel extracts on the lipid profile levels: The effect of selected fruit peel extracts and PHF on total cholesterol (TC), triglycerides (TG), HDL, LDL, VLDL levels was given in Figures 4 and 5. The levels of TC and TG are elevated in disease control (group 2) when compared to control (Group 1) and reduced in peel extracts fed groups (Group 3-12). The levels of LDL and VLDL were significantly reduced in peel extracts fed groups (Group 3-12) when compared to disease control (Group 1). The level of HDL was found significantly high in peel extracts fed groups (Group 3-12) in contrast to disease control (Group 1).
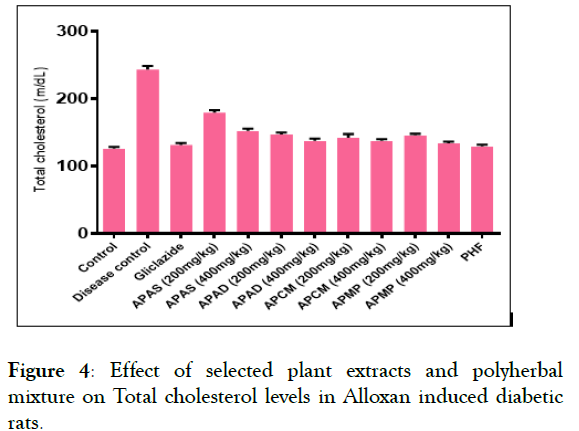
Figure 4: Effect of selected plant extracts and polyherbal mixture on Total cholesterol levels in Alloxan induced diabetic rats.

Figure 5: Effect of selected plant extracts and polyherbal mixture on Triglycerides levels in Alloxan induced diabetic rats.
Histopathological analysis of pancreas by selected peel extracts treatment: The histopathological sections of group 1, 2, 3, 5, 7, 9, 11 and 12 were shown in figure 6. The pancreas of normal rats (group 1) showed normal structure of pancreas with closed and packed acini with connective septa. Within the pancreatic exocrine tissue islets of Langerhans were embedded.

Figure 6: Histopathology of Pancreas
The Alloxan induced diabetic rats (Group 2) showed damaged pancreatic tissue with crowded acini and with less number of cells. It also showed observable cellular infiltrates around some blood vessels, pancreatic ducts and acini.
Examination of pancreas of diabetic animals treated with Gliclazide (Group 3) revealed most of pancreatic acini was closely embedded and dilated ducts with flat epithelial lining. Marked condensation of cellular infiltration was seen around dilated ducts and some acini.
In the pancreas of animals treated with APAS (400 mg/kg) (Group 5) shown pancreatic duct and islet with marked amelioration of damage and necrosis was found. The dose of APAD (400 mg/kg) (Group 7) revealed presence of pale oval islets of Langerhans and packed acini with cellular infiltrates. APCM (400 mg/kg) (Group 9) Observable collagen fibers were also seen around some blood vessels, pancreatic ducts. In APMP (400 mg/kg) (Group 11) treatment shows marked recovery of tubules with packed acini, an islet of langerhans shows exocrine tissue arrangement. Dilated blood vessels were also seen. The PHF treatment group (Group 12) shown islet of pancreas, pancreatic duct, and packed acini.
Alloxan induced diabetogenic activity when administered through parenteral, intravenous, intraperitoneal or subcutaneous route. The induction of diabetes might be due the rapid uptake of alloxan by pancreatic β cells [21] and led to sudden destruction of pancreatic β cells. The alloxan induction led to the stimulation of oxidative stress in the pancreas and reactive oxygen species. A similar uptake of alloxan also takes place in the liver. However, the liver and other tissues are more resistant to reactive oxygen species in comparison to pancreatic β cells and this resistance protects them against alloxan toxicity. In this study we have chosen to evaluate the antidiabetic activity of aqueous peel extracts of four fruits viz., Annona squamosa, Actinidia deliciosa, Cucumis melo, Malus pumila.
In the present investigation, Alloxan induction indicates that the elevated blood glucose levels might be due to the oxidative stress caused and treatment with the selected peel extracts and PHF showed significant reduction in blood glucose levels. The order of potency of selected peel extracts was PHF>APMP>APCM>APAD>APAS in antihyperglycemic activity in alloxan induced rats. In all the selected plant peel extracts and PHF significantly lower the blood glucose, which leads to decrease in the levels of glycosylated hemoglobin. Treatment with the selected peel extracts and PHF showed reduction in the elevated serum lipids such as TG, TC, LDL, VLDL and showed increased. The histopathological studies of the pancreas showed normal structure of pancreas with closed and packed acini with connective septa. Within the pancreatic exocrine tissue islets of Langerhans were embedded.
The Alloxan induced diabetic rats showed damaged pancreatic tissue and with crowded acini with less number of cells. It also showed observable cellular infilatrates around some blood vessels, pancreatic ducts and acini.
Examination of pancreas of diabetic animals treated with Gliclazide revealed most of pancreatic acini was closely embedded and dilated ducts with flat epithelial lining were noticed. Marked condensation of cellular infiltration was seen around dilated ducts and some acini. In the pancreas of animals treated with APAS (400 mg/kg) shown pancreatic duct and islet with marked amelioration of damage and necrosis was found. The dose of APAD (400 mg/kg) revealed presence of pale oval islets of Langerhans and packed acini with cellular infiltrates. APCM (400 mg/kg) Observable collagen fibers were also seen around some blood vessels, pancreatic ducts. In APMP (400 mg/kg) treatment shows marked recovery of tubules with packed acini. An islet of langerhans shows exocrine tissue arrangement. Dilated blood vessels were also seen. The PHF treatment group shown islet of pancreas, pancreatic duct, and packed acini.
The present study showed excellent antidiabetic and hypolipidemic properties of fruit peel extracts in alloxan induced diabetic rats. Histopathological studies of the pancreas indicated that selected peel extracts showed ameliorating effect on damaged pancreas. This might be due to phytochemicals present in the selected peel extracts. The poly herbal mixture of the selected peel extracts showed much more effect compared to that of individual peel extracts. It is concluded that the hypoglycemic action of fruit peel extract may be mediated by more than one biological mechanism including improvement of insulin secretion. Hence further studies are appreciated to establish the mechanism of action and promote the role of poly herbal mixture as a nutraceutical and dietary supplement for diabetics.
The author wish to thank Lavu educational society and Dr. Y. Srinivas Rao, Professor and Principal, Vignan Institute of Pharmaceutical Technology for extending support to carry out this research work.
Citation: Kondepudi GM, Battu GR (2021) Evaluation of Antidiabetic and Hypolipidemic Activities of Selected F ruit Peel E x tracts in Alloxan Induced Diabetic Rats. J Diabetes Meta 12: 865
Received: 26-Apr-2021 Published: 17-May-2021, DOI: 10.35248/2155-6156.21.12.881
Copyright: © 2021 Kondepudi GM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.