Research - (2023) Volume 11, Issue 3
The present study aimed to conduct ethnobiological studies and the antioxidant activity of selected medicinal plants for Diabetes Mellitus management. The field survey was conducted by a semi-structured interview focused on information about respondents and therapeutic habits among traditional health practitioners using the snowball sampling method. The polyphenols and flavonoid contents of various extracts were estimated by the spectrophotometric method. The antioxidant capacity was evaluated by three methods including ABTS, DPPH, and FRAP. A total of 56 traditional health practitioners (39 male and 17 female) were interviewed wherein the age range of 51 years to 60 years old has the highest frequency (35.73%). As to marital status, the majority of informants (92.85%) were married and most of them were illiterate (82.14%). A total of 28 medicinal plants were reported belonging to 18 families of which the Fabaceae family (7 species) was the most represented. The species Phyllanthus amarus (26.24%), Chrysanthellum americanum (24%), Striga hermonthica (20%), Chamaecrista nigricans (18.69%), Leptadenia hastata (17.67%) and Detarium microcarpum (12.36%) were the most cited. The leaves (46%) and the whole plants (39%) were the most commonly used parts. The decoction (73.21%) and the drink (87.5%) were respectively the extraction method and the administration mode. The best content of polyphenols was obtained by the ethyl acetate fraction of P. amarus (34.84 mg ±1.23 mg GAE/100 mg fraction) while that of flavonoids was obtained by the ethyl acetate fraction of S. hermonthica (15.77 µmol AAE/g ± 1.33 mg QE/100 mg fraction). As the antioxidant activity, methanolic extracts of P. amarus presented significant activities of ABTS (11418.70 µmol AAE/g ± 291.45 µmol AAE/g extract), DPPH (488.546 µmol AAE/g ± 0 µmol AAE/g extract) and FRAP (1348.74 µmol AAE/g ± 166.85 µmol AAE/g extract). The findings results might be used as baseline information for further scientific investigation to develop new antidiabetic plant-based drugs.
Ethnobotany • Medicinal plants • Diabetes • Polyphenols • Antioxidant
Ethnobotany is a multidisciplinary activity that includes basic documentation of traditional botanical knowledge, quantitative assessment of botanical reso-urce use and management, experimental evaluation of benefits derived from plants, and finally, implementation of projects to increase the value that local people obtain [1,2]. Currently, ethno medical studies are crucial for the discovery of new plant-based medicines from indigenous plant species [3-5]. In many parts of the world, medicinal plants have been widely used for their therapeutic properties, and literature on their use exists [3,6,7]. In West African countries, including Burkina Faso, traditional medicine and pharmac opeia are still the main primary healthcare resource for about 70% of the population [8,9].
Oxidative stress is defined as a loss of balance between oxidants and antioxidants inside a cell [10,11]. It is implicated in the dysregulation of βcells during diabetes, due to the weakness of their antioxidant protection systems [12]. Numerous compounds derived from natural plant sources have been identified as scavengers of free radicals or active oxygen [13,14].
Phyllanthus amarus (Phyllanthaceae) are widespread in tropical and subtropical countries of Africa, Asia, South America, and the West Indies [15]. Phytochemical investigations of P. amarus have revealed interesting compounds of medicinal importance including lignans, flavonoids, hydrolyzable tannins, polyphenols, triterpenes, sterols, and alkaloids. The extracts, as well as the isolated compounds from P. amarus , have been reported in various biological activities such as antioxidant, antidiabetic, hypolipidemic, hepatoprotective, anti-inflammatory, anticancer, nephroprotective, antiviral, antibacterial, antiplasmodial, antimalarial, antimicrobial, and diuretic [15-17].
Chrysanthellum americanum (Asteraceae) is a small, upright, or low-lying herb with a few leaves and yellow flowers [18,19]. It is used in the treatment of fever, hepatitis, jaundice, urinary lithiasis, dysentery, and swollen legs [20-22]. The majority of the biological properties of the extracts of this plant are related to saponins and flavonoids [20-24].
Striga hermonthica (Delile) Benth. (Orobanchaceae) are a semi-parasitic herb that grows in millet (Pennisetum americanum ) and sorghum (Sorghum bicolor ) crops and is widely distributed in West and East Africa [25-27]. This plant has been used in traditional medicine to treat dermatitis, ulcers, leprosy, pneumonia and jaundice, as a trypanocidal, antibacterial, and antiplasmodic [28-32]. Phytochemical studies conducted on methanolic extracts of S. hermonthica leaves revealed the presence of alkaloids, saponins, cardiac glycosides, tannins, flavonoids, carbohydrates, and phenols [33].
To the best of our knowledge, no ethnobotanical studies related to diabetes has been reported and published in this area of Burkina Faso. This study aims to document the traditional of locals knowledge regarding medicinal plants used for diabetes management and to assess the antioxidant capacity of selected medicinal plants.
Study area
Located in the southwestern part of Burkina Faso, in the Hauts-Bassins region, Bobo-Dioulasso is a city covering an area of 136.78 km2 . With a population of 1,509,377, the geographic coordinates are 11°11'00'' north and 4°17'00'' west.
The climate is of the South Sudanese type and is characterized by a dry season (October to April) and a rainy season (May to September). The dry season is marked by a cold period (November to January) and a hot period (February to April). The vegetation is of the South Sudanese type, consisting of wooded savannahs, tree and shrub savannahs. Annual rainfall is between 800 mm to 1200 mm [34] (Figure 1).
Figure 1: Map showing study area.
Ethnobotanical survey
An ethnobotanical survey on medicinal plant recipes used in the treatment of diabetes was conducted among 56 traditional health practitioners in the city of Bobo-Dioulasso. The survey took place from May to July 2019 (03 months) and participants were selected among the traditional practitioners identified in the markets or organized in associations using the snowball sampling method. The techniques used were direct and individual interviews to avoid the answers obtained from one traditional practitioner being influenced by the other. The tools used were a guide sheet with a specially prepared and pre-tested semi-structured questionnaire. The questionnaire focused on information about respondents, the therapeutic habits of the population in the management of diabetes, in particular the medicinal plants (local name) used to treat diabetes, the parts of the plants used, the methods of preparation, and the mode of administration. Informed consent from all participants was verbally obtained before conducting interviews, and ethical guidelines prescribed by the International Society of Ethnobiology were followed [35].
Solvents and reagents
The Folin-Ciocalteu reagent, NaH2PO4, Na2HPO4, sodium carbonate, aluminum trichloride, gallic acid, and quercetin were purchased from SigmaAldrich Chemie, Steinheim, Germany. 2,2-Diphenylpicrylhydrazyl (DPPH), 2,2'-azinobis (3-ethylbenzothiazoline)-6-sulfonic (ABTS), trichloroacetic acid, and solvents used were from Fluka Chemie, Switzerland. Potassium hexacyanoferrate [K3Fe(CN)6] was supplied by Prolabo and ascorbic acid by Labosi, Paris, France. The solvents used were of analytical grade
Plant material and preparation of extract
For the collection, whole plants of Striga hermonthica, Chrysanthellum americanum, and Phyllanthus amarus were collected in August 2019 respectively in Dinderesso (village distant 16 km from Bobo-Dioulasso N 11°15'04.9''; W 004°26'08.6''), Dar es salam (village distant about 20 km from Bobo-Dioulasso N 11°00'46.4''; W 004°20'39.6'') and in Bama (village 25 kilometers from Bobo-Dioulasso N 11°22'31.3''; W 004°23'51.5'') in fallow lands. A previous collection authorization was issued by the forestry agents. Specimens collected were identified by Dr. Hermann Yempabou Ouoba, Botanist and Phytoecologist at Joseph Ki-Zerbo University whereas the International Plant Name Index (www.ipni.org) was used to obtain the correct botanical name confirmation.
Samples were rinsed and dried in the shade under laboratory conditions. They were then pulverized with an aluminum mortar and packaged in labeled ZIP bags until use. For extraction, 15 g of plant powder from each sample (a total of 30 g extracted per sample) was weighed and loaded into extraction cartridges and placed in a soxhlet (Behrotest, Germany). A volume of 200 mL of methanol was put into the extraction flask and the temperature was maintained at 70°C. This operation lasted at least 4 hours for each sample and then the extract was concentrated and placed in empty Petri dishes previously labeled and weighed. These crude extracts are then deposited in the open air to evaporate the solvent. The methanol extracts (EMeOH) were subjected to sequential liquid-liquid extraction with dichloromethane (FDCM) and ethyl acetate (FAE). Each fraction was then collected, concentrated, and placed in empty Petri dishes previously labeled and weighed to evaporate the solvent.
Determination of total polyphenols
The total phenolic contents were done using the method described by [36]. The 10 mg/mL stock solution of the crude extracts of each sample and fraction was diluted to the hundredth with distilled water to have a final concentration of 100 µg/mL. To a volume of 125 µL of the diluted solution of each extract or fraction was added 625 µL of foling-ciocalteu reagent (FCR; 0.2N). After 5 minutes of incubation, 500 µL of sodium carbonate at 75 g/L was added and the whole mixture was incubated in the dark for 2 hours. Absorbances and concentrations are read from a spectrophotometer against a blank containing only distilled water at 760 nm. In total, 3 readings are taken for each extract or fraction and the result given is an average obtained from the three values. Total phenolic contents are determined using a reference curve (y=4.668 10-3x-0.034; R2=0.9991) based on gallic acid (0 mg/L-200 mg/L). The results are expressed as mg Gallic Acid Equivalent (mg GAE)/100 mg extract or fraction.
Detemination of total flavonoids
The total flavonoïds content was done using the method described by [36]. To a volume of 625 µL of the diluted solution was added 625 µL of Aluminum Chloride (2% AlCl3) and the mixture was incubated for 10 minutes in the dark. The blank was prepared with 625 µL of the diluted solution added to 625 µL of methanol. Absorbances and concentrations were read from a spectrophotometer at a wavelength of 415 nm. A total of 3 readings were taken for each extract or fraction and the result given is an average obtained from the three values. Total flavonoid contents are determined using a reference curve (y=1.229 10-2 x; R2=0.9990) established from quercetin (0 mg/L-50 mg/L). Results are expressed as mg Quercetin Equivalent (mg QE)/100 mg extract or fraction.
Antioxydant activities
Iron (III) to iron (II) reduction activity (FRAP): The FRAP assay was done using the method described by [36]. Stock solutions (10 mg/mL) were diluted to the hundredth in distilled water to have a final test concentration of 100 µg/mL. In each of the 3 test tubes, 0.5 mL of the diluted solution was introduced, and 0.5 mL of distilled water in a fourth tube for the blank. To these different tubes, 1.25 mL of phosphate buffer (0.2 M; pH 6.6) and then 1.25 mL of potassium hexacyanoferrate [K3Fe(CN)6] were added successively. The mixture was heated in a water bath at 50°C for 30 minutes, then a volume of 1.25 mL of trichloroacetic acid (10%) was added and the mixture was centrifuged at 3000 rpm for 10 min. A 0.625 mL of the supernatant from each tube was added to tubes containing 0.625 mL of distilled water, and then a volume of 125 µL of freshly prepared Trichloroferrate [FeCl3 (0.1%)] was added to the resulting mixture. All of the resulting solutions were shaken and then run on a spectrophotometer for a series of 3 absorbance and concentration readings at a wavelength of 700 nm against a standard (y=3.270 10-3 x; R2=0.9990) established from ascorbic acid (0 µg/mL-10 µg/mL).
ABTS radical cation decolorization assay: The radical scavenging capacity of antioxidants for the ABTS (2,2’-azinobis-3-ethylbenzothiazoline-6- sulphonate) radical cation was determined as described [36]. Stock solutions (10 mg/mL) were diluted to the hundredth in distilled water to have a final test concentration of 100 µg/mL. A volume of 10 μL of the diluted solution was added to 990 μL of the fresh ABTS+solution and then the whole set was incubated in the dark for 15 minutes. Absorbances and concentrations were read 3 times at a wavelength of 734 nm on a spectrophotometer against a standard curve (y=-7.874 10-4 x +0.709; R 2=0.9993) made from ascorbic acid (0 µg/mL-10 µg/mL).
DPPH radical scavenging activity: The ability of the extract to scavenge the radical DPPH (2,2-diphenyl-1-picrylhydrazyl) was evaluated as described by [36]. The different stock solutions of extracts (10 mg/mL) and fractions (10 mg/mL) were diluted to the hundredth in methanol to have a test concentration of 100 µg/mL. In each of the 3rd test tubes, 375 µL of the diluted solution and 750 µL of a DPPH solution (20 mg/L) were introduced and the whole mixture was incubated for 15 minutes in the dark. A blank was prepared with 375 µL of the extract or fraction added to 750 µL of methanol. Absorbances and concentrations were read using a spectrophotometer at 517 nm against a standard (y=-2.224 102 x + 0.348; R2=0.9966) obtained from ascorbic acid (0 µg/mL-10 µg/mL)
Statistical analysis
The results of the experiments are the mean ± SD of three measurements performed in parallel. The QGIS 3.22 software was used to plot the map. Pearson test was performed by the software Statat 13.0 to compare the means of each plant extract or fraction, quercetin was used as a reference. p-values (p<0.05) were considered significant.
Ethnobotanical survey
Demographic characteristics of traditional health practitioners of BoboDioulasso were gathered through a semi-structural interview (Table 1). A total of 56 traditional health practitioners were interviewed wherein 69.64% were male and 30.35% female. In terms of age, among the respondents, the age range of 51 years to 60 years old 35.73% has the highest frequency of interviews, followed by 41 years to 50 years old 28.57%, 31 years to 40 years old 16.07%, both 21 years to 30 years old, ≥ 61 years old 8.92% and ≤ 20 years old 1.79%. As to marital status, 92.85% were married and 7.14% were single. The majority of informants were illiterate (82.14%), some had basic studies (16.07%) and high school education (1.79%) was less cited.
Table 1. Demographic profile of traditional health practitioners
Demographic characteristics |
Traditional health practitioners of Bobo-Dioulasso | Percentage |
|---|---|---|
Sex |
Number of male and female informants | |
Male |
39 | 69.64% |
Female |
17 | 30.35% |
Total |
56 | 100% |
Age range |
Number of respondents by age group | |
≤ 20 years |
1 | 1.79% |
21 years-30 years |
5 | 8.92% |
31 years-40 years |
9 | 16.07% |
41 years-50 years |
16 | 28.57% |
51 years-60 years |
20 | 35.73% |
≥ 61 years |
5 | 8.92% |
Total |
56 | 100% |
Marital status |
Number of respondents by marital status | |
Single |
4 | 7.14% |
Married |
52 | 92.85% |
Total |
56 | 100% |
Education level |
Number of respondents by education level | |
Illiterate |
46 | 82.14% |
Basic studies |
9 | 16.07% |
High school |
1 | 1.78% |
University |
0 | 0% |
Total |
56 | 100% |
An ethnobotanical survey including plant species is summarized in Figure 2. A total of 28 medicinal plants were reported of which the most cited were Phyllanthus amarus (26.24%), Chrysanthellum americanum (24%), Striga hermonthica (20%), Chamaecrista nigricans (18.69%), Leptadenia hastata (17.67%), Detarium microcarpum (12.36%), Combretum microcarpum (11.43%), Ficus ingens (10.10%) and Gardenia erubensis (10.10%).
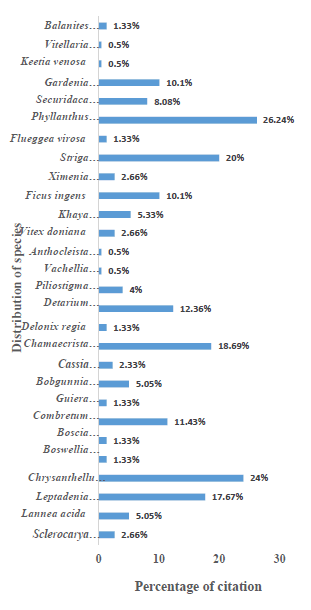
Figure 2: Frequency of citation of medicinal plants used for diabetes care at BoboDioulasso.
Several species were cited in this study offering a wide range of medicinal plants. Indeed, plant biodiversity is a potentially valuable source of novel drugs [37].
The number of family use importance results is presented in Figure 3. In the current study, eighteen families were reported among which the dominant family was Fabaceae (7 species), followed by Anacardiaceae, Combretaceae, Phyllanthaceae, and Rubiaceae (2 species each). Similarly, our results show that out of the 18 families, Fabaceae, Rubiaceae, Apocynaceae, Asteraceae, and Lamiaceae are among the families that have been previously reported by [38] as the most used plant families in Burkina Faso. Consequently, in this study, the Fabaceae family regrouped the largest number of medicinal plant species. The results are in line with those found by [38], which reported that the Fabaceae family is among the most species-rich families in traditional medicine in Burkina Faso.
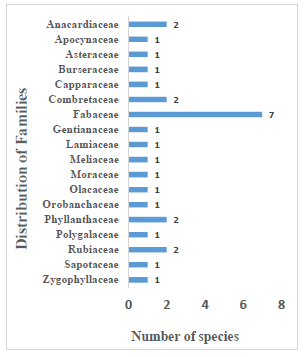
Figure 3: Distribution of species across different families of medicinal plants.
The distribution of plant parts used that the leaves (46%) and the whole plant (39%) were the most used in this study (Figure 4). The roots bark (8%), trunk bark (6%) and seeds (1%) were less used. The use of the aerial parts could be explained by easy access. Some authors encourage the use of leaves because they are a source of synthesis of secondary metabolites and their use would ensure the sustainable management of medicinal plants [39-41].
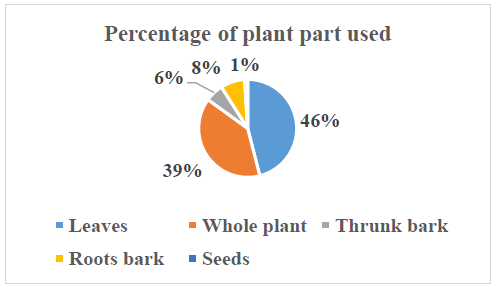
Figure 4: Distribution of plant part used.
Various methods of preparation of medicinal plants and modes of drug administration were used by traditional health practitioners (Tables 2 and 3). As a method of preparation, decoction was preferable (73.21%), followed by infusion (16.07%). The maceration (7.14%) and powder (3.57%) were less used. Regarding drug administration, drinking was essentially (87.5%) used. Bath (7.14%), chewing (3.57%) and purge (1.78%) were less cited.
Table 2. Methods of preparation of medicinal plants
Method of preparation |
Number of citation | Percentage |
|---|---|---|
Decoction |
41 | 73.21% |
Maceration |
4 | 7.14% |
Infusion |
9 | 16.07% |
Powder |
2 | 3.57% |
Total |
56 | 100% |
Table 3. Modes of drug administration.
Mode of administration |
Number of citation | Percentage |
|---|---|---|
Drink |
49 | 87.50% |
Bath |
4 | 7.14% |
Chewing |
2 | 3.57% |
Purge |
1 | 1.78% |
Total |
56 | 100% |
Total polyphenols and flavonoids estimation
The results of polyphenol content ranged between 7.06 mg ± 0.37 mg to 35.56 mg ± 0.65 mg GAE/100 mg extracts/fractions while the flavonoids content ranged between 1.80 ± 0.12 to 15.77 mg ±1.33 mg QE/100 mg extracts/fractions (Table 4). The FAE of P. amarus (34.84 mg ±1.23 mg GAE/100 mg fraction) had the significant total polyphenols content. In terms of flavonoid content, the best flavonoid content was presented by the FAE of S. hermonthica (15.77 mg ±1.33 mg QE/100 mg fraction).
Table 4. Total polyphenols and total flavonoid content
Plants extracts/Fractions |
Total polyphenols (mgGAE/100 mg extracts/fractions) | Total flavonoids (mgQE/100 mg extracts/fractions) |
|---|---|---|
P. amarus |
||
EMeOH |
35.56 ± 0.65 | 3.81 ± 0.34 |
FDCM |
20.35 ± 3.53 | 7.06 ± 1.11 |
FAE |
34.84 ± 1.23*** | 1.80 ± 0.12 |
C. americanum |
||
EMeOH |
18.06 ± 0.12 | 5.13 ± 0.37 |
FDCM |
9.13 ± 3.02 | 7.06 ± 0.37 |
FAE |
17.63 ± 0.32 | 3.65 ± 0.07 |
S. hermonthica |
||
EMeOH |
30.78 ± 0.12 | 8.65 ± 0.13 |
FDCM |
14.48 ± 1.88 | 12.42 ± 0.77 |
FAE |
29.42 ± 3.09*** | 15.77 ± 1.33 |
Different values are mean ± SD (n=3) and indicate in the same column significant difference: *** (p<0.05); GAE: Gallic Acid Equivalent; QE: Quercetin Equivalent. EMeOH Methanolic extract; FDCM: Dichloromethane fraction; FAE: Ethyl acetate fraction |
||
Antioxidant activities
The antioxidant capacity was performed by three methods ABTS, DPPH, and FRAP described by and using the quercetin as a reference [36] (Table 5). The antiradicalaire activity by the ABTS method was used to determine the ability of various extracts or fractions to scavenge the free radical. The methanolic extracts and the ethyl acetate fraction of both plants P. amarus (EMeOH: 11418.70 µmol AAE/g ± 291.45 µmol AAE/g extract; FAE: 10240.77 µmol AAE/g ± 260.02 µmol AAE/g fraction) and S. hermonthica (EMeOH: 9134.96 µmol AAE/g ± 110.16 µmol AAE/g extract; FAE: 9231.11 µmol AAE/g ± 249.81 µmol AAE/g fraction) were significantly different, quercetin was used as reference (p<0.05). The antioxidant capacity evaluated by DPPH radical scavenging method was presented both plants P. amarus (EMeOH: 488.546 ± 0 µmol AAE/g extract; FAE: 471.51 µmol AAE/g ± 5.11 µmol AAE/g fraction) and S. hermonthica (EMeOH: 136.17 µmol AAE/g ± 9.67 µmol AAE/g extract; FAE: 360.02 µmol AAE/g µmol AAE/g ± 8.94 µmol AAE/g fraction) as significantly different, quercetin was used as reference (p<0.05). Concerning the FRAP method which allows measuring the fitness of plant extracts or fractions to reduce the iron Fe (III) to Fe (II), the EMeOH (1348.74 ± 166.85) of P. amarus presented a significant difference, quercetin was used as reference (p<0.05).
Table 5. Total antioxidant capacity.
Plants extracts/Fractions |
ABTS (µmol AAE/g extract or fraction) | DPPH (µmol AAE/g extract or fraction) | FRAP (µmol AAE/g extract or fraction) |
|---|---|---|---|
P. amarus |
|||
EMeOH |
11418.70 ± 291.45*** | 488.546 ± 0*** | 1348.74 ±1 66.85*** |
FDCM |
10937.91 ± 333.09 | 451.92 ± 3.87 | 602.00 ± 43.69 |
FAE |
10240.77 ± 260.02*** | 471.51 ± 5.11*** | 1881.28 ± 182.40 |
C. americanum |
|||
EMeOH |
9471.51 ± 110.16 | 142.98 ± 5.90 | 0 ± 0*** |
FDCM |
8149.34 ± 288.47 | 131.06 ± 3.90 | 0 ± 0*** |
FAE |
7428.17 ± 1.11E-12 | 470.66 ± 15.40 | 793.03 ± 60.99 |
S. hermonthica |
|||
EMeOH |
9134.96 ± 110.16*** | 136.17 ± 9.67*** | 347.3 ± 0 |
FDCM |
9735.95 ± 0 | 175.30 ± 2.96 | 0 ± 0*** |
FAE |
9231.11 ± 249.81*** | 360.02 ± 8.94*** | 196.82 ± 10.02 |
Reference Quercetin |
14474.73 ± 213.43 | 645.58 ± 3.20 | 5991.29 ± 75.56 |
Different values are mean ± SD (n=3) and indicate in the same column significant difference: ***(p<0.05) ; AAE: Ascorbic Acid Equivalent |
|||
In this study, all three medicinal plants showed antioxidant activities. Previous studies have shown that the compounds isolated from P. amarus exhibited interesting antioxidant activities through the three methods namely DPPH, ABTS and FRAP [42]. To this effect, phyllanthin showed high antioxidant capacity with an IC50 of 7.4 µmol/mL by DPPH method [43]. Similarly, C. americanum extracts showed interesting in vitro antioxidant capacity with inhibition values of 35.01% ± 0.26% of LPO and 42.01 mg TE/g ± 0.26 mg TE/g through the TEAC method. In 2005, the antioxidant capacity of S. hermonthica was evaluated by the DPPH method. The results showed a low antioxidant capacity of the acetone extracts (with an IC50 of 95.27 µg/mL ± 2.30 µg/mL) against a high free radical scavenging capacity of Luteolin (IC50 of 6.80 µg/mL ± 1.46 µg/mL), a compound isolated from the ethyl acetate fraction [26] (Table 6).
Table 6. Medicinal plants used by traditional health practitioners for diabetes care at Bobo-Dioulasso.
Family/Scientific name |
Local name | Habit of Growth | Used part | Method of Preparation | Mode of administration |
|---|---|---|---|---|---|
Anacardiaceae |
|||||
Sclerocarya birrea Hochst. |
Demissin doro (Dioula) | Tree | Leaves, Trunk bark, | Decoction, maceration | Drink |
Lannea acida A.Rich. |
Kuna yiri (Dioula) | Tree | roots bark | Decoction | Drink |
Apocynaceae |
|||||
Leptadenia hastata (pers.) Decne |
Sowe (Dioula) | Herb | The whole plant, leaves | Decoction | Drink |
Asteraceae |
|||||
Chrysanthellum americanum Vatke |
Waltuko (Moore) | Herb | The whole plant, leaves | Decoction, infusion | Drink |
Burseraceae |
|||||
Boswellia dalzielii Hutch. |
Gondregnogo (Moore) | Tree | Trunk bark | Decoction | Drink |
Capparaceae |
|||||
Boscia senegalensis Lam. |
Bere (bambara) | Shrub | Leaves | Decoction | Drink |
Combretaceae |
|||||
Combretum micranthum G.Don |
Kinkeliba (Dioula) | Shrub | Leaves, seeds, roots | Decoction, maceration | Drink, bath, chewing |
Guiera senegalensis J.F.Gmel. |
Koun goue (Dioula) | Shrub | Roots, bark | Decoction | Drink |
Fabaceae |
|||||
Bobgunnia madagascariensis (Desv.) J.H.Kirkbr. & Wiersema |
Sindjan goue (Dioula) | Tree | Roots, bark | Decoction, Powder | Drink |
Cassia sieberiana DC. |
Sindjan (Dioula) | Tree | Roots, bark | Decoction, Powder | Drink, bath |
Chamaecrista nigricans (Vahl) Greene |
Douguouma djalan (Dioula) | Herb | The whole plant, leaves | Decoction | Drink |
Delonix regia (Bojer ex Hook.) Raf. |
Toubabounere (Dioula) | Tree | Leaves | Decoction | Drink |
Detarium microcarpum Guill. & Perr. |
Kagdga (Moore) | Tree | Leaves | Decoction | Drink |
Piliostigma thonningii (Schumach.) Milne-Redh. |
Bangde (moore) | Tree | Fruts | Chewing | |
Vachellia nilotica (L.) P.J.H.Hurter & Mabb. |
Bagana (Moore) | Tree | Leaves | Decoction | Drink |
Gentianaceae |
|||||
Anthocleista procera Lepr. ex Bureau |
Farata-debe (Dioula) | Tree | Leaves | Decoction | Drink |
Lamiaceae |
|||||
Vitex doniana Sweet |
Koto (Dioula) | Tree | Trunk bark, roots bark | Decoction, infusion | Drink |
Meliaceae |
|||||
Khaya senegalensis A.Juss. |
Djalan (Dioula) | Tree | Trunk bark, roots bark | Decoction, infusion | Drink, |
Moraceae |
|||||
Ficus ingens Miq. |
Kuinkuiga (Moore) | Tree | Root bark | Decoction, infusion | Drink |
Olacaceae |
|||||
Ximenia americana L. |
Mini guani (Dioula) | Shrub | Roots bark | Decoction | Drink |
Orobanchaceae |
|||||
Striga hermonthica (Delile) Benth. |
Seguin (Dioula) | Herb | The whole plant, leaves | Decoction, infusion | Drink |
Phyllanthaceae |
|||||
Flueggea virosa (Roxb. ex Willd.) Royle |
Balan balan (Dioula) | Shrub | Leaves | Decoction | Drink |
Phyllanthus amarus Schumach. & Thonn. |
Denbambou (Dioula) | Herb | The whole plant, leaves | Decoction | Drink |
Polygalaceae |
|||||
Securidaca longepedunculata Fresen. |
Pelga (Moore) | Shrub | Roots bark | Decoction | Drink |
Rubiaceae |
|||||
Gardenia erubescens Stapf & Hutch. |
Subudga (Moore) | Shrub | Roots, bark | Decoction, maceration | Drink, bath |
Keetia venosa (Oliv.) Bridson |
Ladji fofana (dioula) | Shrub | Leaves | Decoction | Drink |
Sapotaceae |
|||||
Vitellaria paradoxa C.F.Gaertn. |
Sii yiri (Dioula) | Tree | Trunk bark | Decoction | Drink |
Zygophyllaceae |
|||||
Balanites aegyptiaca (L.) Delile |
Zeguene (bambara) | Shrub | Roots bark | Decoction | Drink |
The ethnobotanical findings documented in this study provide evidence of the use of medicinal plants for diabetes management by the traditional health practitioners of Bobo-Dioulasso. P. amarus, C. americanum, S. hermonthica, C. nigricans, L. hastata and D. microcarpum were the most cited whereas leaves and whole plants the most used. The decoction and drink were used for drug preparation and administration. P. amarus, C. americanum, S. hermonthica, contain different classes of organic compounds which are responsible for antioxidant activity. However, further scientific investigations are required to establish the anti-diabetic effects of these plants.
Acknowledgment
The authors are grateful to all the traditional healers of Bobo-Dioulasso who took part in this study by sharing their herbal recipes.
Ethical statement
The research was conducted following the Code of Ethics of the International Society of Ethnobiology (ISE, 2006).
Conflict of interests
The authors declare no potential conflict of interest in this manuscript.
Author’s contributions
• Segda: Conceptualization, Methodology, Investigation, Formal analysis, Writing-original draft;
• N. T. R. Meda: Conceptualization, Methodology, Writing-review & editing, Validation;
• M. J. Bangou: Conceptualization, Methodology;
• K. Koama: Writing - review and editing;
• H. Y. Ouoba: Investigation, Writing-review, and editing;
• W. Kagambega: Writing-review and editing;
• S. E. Kam: Writing-review and editing;
• G. A. Ouedraogo: Supervision; Visualization, Validation.
All authors consent to publish this manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Citation: Segda, A., et al. Ethnobotany of Medicinal Plants for Diabetes and Antioxidant Activity of Selected Phyllanthus amarus Schum. and Thonn., Chrysanthellum americanum (L.) Vatke. and Striga hermonthica (Delile) Benth. of Burkina Faso. Nat Prod Chem Res. 2023, 11(3), 1-7.
Received: 11-May-2023, Manuscript No. npcr-23-24059; Editor assigned: 12-May-2023, Pre QC No. npcr-23-24059(PQ); Reviewed: 20-Jun-2023, QC No. npcr-23-24059(Q); Revised: 25-Jun-2023, Manuscript No. npcr-23-24059(R); Published: 30-Jun-2023, DOI: 10.35248/2329-6836.23.11.3.1-7
Copyright: �©2023 Segda A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.