Research Article - (2021) Volume 12, Issue 8
Introduction: Diabetic foot ulcers are one of the most serious complications for patients with diabetes mellitus. The use of nutritional supplements such as Diamel® can be considered as an adjunct to conventional treatment in affected patients.
Objective: To evaluate the effectiveness of the product Diamel® in the treatment of diabetic foot ulcers.
Methods: Phase II double-blind, randomized, placebo-controlled clinical trial in a sample of 100 patients of both sexes with type 2 diabetes mellitus (DM) and with Wagner grade I-II-foot ulcers. All of them received conventional therapy, 50 of them combined with the product Diamel® and 50 combined with placebo. They followed up for a period of 1 year. The proportions of healed patients were taken as a response variable to determine efficacy. Other variables are analyzed: body mass index, glycemia, glycosylated hemoglobin, cholesterol, triglycerides, and creatinine, which were compared at the beginning and end of the study. Data analysis was performed according to the intention-to-treat principle.
Results: The Diamel® group showed a 1-year cumulative healing rate of 89.2% (95% CI, 77.4-96.5) vs 72.4% (95% CI, 58.0-85.1) in the placebo group. The average healing time for ulcers in the Diamel® group was 3 months, significantly less than the Placebo group, which took 5 months, p=0.001. At the end of the study, HbA1c and fasting blood glucose had decreased significantly in the Diamel® group. Recurrence rates were lower in the Diamel® group (3 patients, 8%) than in the placebo group (8 patients, 16%), and no major adverse effects were reported in relation to the supplement.
Conclusions: The Diamel® nutritional supplement showed a sufficient degree of efficacy in the treatment of diabetic foot ulcers in patients with type 2 DM, so large-scale studies should be undertaken.
Diabetic foot ulcer; Diamel® nutritional supplement; Randomized phase II trial; Type 2 diabetes mellitus
A diabetic foot ulcer (DFU) is one of the most serious chronic complications of diabetes mellitus (DM) and is the cause of most non-traumatic lower extremity amputations [1]. According to epidemiological studies, 15% of people with DM will develop foot ulcers at some point in their lives, which could impair their quality of life [2-8].
The Cuban health system’s actions for people with DM consist of prevention and timely treatment of complications in order to prevent ulceration, amputation, and disability. As soon as a foot lesion develops, prompt and efficient care is required. Good metabolic control and adequate vitamin and nutritional intake as part of the comprehensive management of affected patients all play an important role in the progression of DFU [9-16].
Nutritional supplements are advantageous because they are inexpensive, have few adverse effects, and can be applied in large populations [17,18]. Encouraging results have been achieved with nutritional supplements such as Diamel®, which is registered in Cuba by the National Institute of Food Hygiene (INHA).
Diamel® is a nutraceutical product containing trace elements, amino acids, vitamins, and lettuce and blueberry extracts. It is taken orally and shows few adverse effects (Annex 1). During the product’s manufacturing process [19], its components are activated by a magnetization or molecular activation process, which improves the properties of said elements. Diamel’s mechanism of action takes place at the pancreatic, gastrointestinal, and intracellular levels [19, 20-24]. The use of Diamel has been shown to decrease insulin resistance in people suffering from metabolic syndrome [25] and in women with polycystic ovary syndrome [26]. Its natural ingredients can act as biocatalysts and antioxidants, and lettuce extract may decrease gastrointestinal glucose absorption [19, 20, 25, 26].
Considering this background, we set out to explore whether treatment with Diamel® combined with conventional therapy in patients with type 2 DM treated with insulin could increase the healing rate of low-severity diabetic foot ulcers without arterial involvement or with mild arterial involvement in order to propose Phase III studies.
A phase II clinical trial was carried out with 100 patients who consecutively attended the diabetic foot clinic of the Havana Diabetic Care Center (CAD). The clinical trial was randomized, placebo-controlled, and double blind. The patients, of both sexes, had type 2 diabetics (DM2) and were treated with insulin. They had been diagnosed with Wagner grade I-II neuropathic foot ulcers [27] based on the pressure index (PI). The clinical trial was registered in the international registry of clinical trials (United States National Library of Medicine, ClinicalTrials.gov, under code: NCT03583593. Website: https://clinicaltrials.gov/ct2/show/NCT03583593
Inclusion criteria
Adult subjects with insulin-treated type 2 DM with neuropathic diabetic foot ulcers, without evident infection, with Wagner grade I and II severity, and without arterial disease or with mild arterial disease found on physical examination and pressure index determined by MiniDoppler equipment.
Exclusion criteria
Patients requiring treatment with Heberprot-P or surgery, with evident infection of the ulcer tissue, with a history of anemia or another chronic debilitating or psychiatric disease (with cognitive limitations); pregnant women and patients with hypersensitivity to any of the product’s components or those under treatment with steroids or immune-suppressants.
Interventions and assessment
Patients were recruited consecutively, according to the inclusion criteria. Treatment (Diamel or Placebo) was randomized. To ensure blinding, identical labels were used for both treatments. Two therapeutic groups were thus formed:
Diamel group: This group received 3,960 mg of Diamel® on a daily basis, at a dose of two 600 mg capsules three times a day, just before breakfast, lunch, and dinner, plus conventional therapy.
Placebo group: This group received 600 mg of Diamel placebo, at a similar dose and frequency, plus conventional therapy.
The product under evaluation (PE) is the nutritional supplement Diamel®, whose characteristics are described in Annex 1 (Annex 1). It was supplied by the Spanish laboratory Catalysis, S.L. and marketed as a nutritional supplement by said laboratory (Macarena, no. 14, 28016 Madrid, Spain). In Cuba, the regulatory institution for nutritional supplements and for authorizing their use in clinical trials is the Institute of Nutrition and Food Hygiene (INHA). This product was registered with the following healthcare license: PI-5513/15 (2015) Renewed on: 1/10/2018 PI-R20802/18 (valid for three years); Volume XXI Page 802.
Both groups treated with the conventional therapy protocol for these types of ulcers: treatment with insulin for glycemic control, hygiene/dietary and foot care measures, and local topical ulcer treatment every other day.
Indications and treatment were individualized in consultations within the CAD (Havana Diabetic Care Center). Patients were evaluated in the diabetic foot consultation monthly during the first six months. They were also followed up at nine months and, ultimately, at 12 months, when the final evaluation was performed. Patients were required to hand in the empty bottles consumed during the period at each consultation to ensure adherence to the product. At each consultation, a general and local physical examination of the lower limbs was performed to describe the evolution of the ulcerative lesion and the possible appearance of any new lesions. Treatment lasted 12 months after inclusion. Quarterly blood controls of glycemia, glycosylated hemoglobin (HbA1c), cholesterol, triglycerides, and creatinine were performed. The data collected was in a case report form designed for this purpose.
Overall study definitions
According to the pressure index (PI), the following limits defined as:
• No arterial disease: PI ≥ 1.0
• Mild arterial disease: 0.8 ≤ PI ≥ 0.9.
Healed: When the ulcer was completely re-epithelialized.
Conventional therapy: Treatment with insulin, hygiene/dietary guidelines, foot care and local ulcer treatment.
The department’s Procedures Manual [28] currently recommends these measures.
Explanatory variables: Sex, age, pressure index (PI), ulcer location, affected limb, smoking habit, duration of DM, insulin dose, body mass index (BMI), blood pressure, blood glucose, cholesterol, triglycerides, and glycosylated hemoglobin (HbA1c).
Primary response variable: Proportion of patients achieved healing in terms of complete ulcer closure or epithelialization.
Secondary response variables: Glucose concentration, cholesterol, fasting triglycerides, HbA1c concentration, takes time to heal.
Adverse reactions: During each visit, participants were questioned and clinically examined to check if they are experienced any adverse effects such as skin rashes, headaches, diarrhea, nausea, dyspepsia, and inflammatory disease.
Statistical procedures
Sample size calculation: The one-stage designs proposed by A’Hern [29] without early stopping rules used to obtain the sample size. It was decided that the combination of Diamel+ conventional treatment would clearly be declared ineffective if the success rate (P) was ≤ 15% (p0), meaning the level of success below which the product shows no signs of efficacy (the study does not warrant further investigation). In addition, the minimum required efficacy value (p1) was set at 30%, above which the product would be declare effective (the results warrant proceeding to a Phase III study).
Errors α= 5% and β= 20% were set, yielding 48 (rounded to 50) as the number of subjects to be recruited. The number of responses (a) is set at 12, so the product will be declare ineffective if the responses do not exceed this number. In addition, r = a+1, that is, the number of responses where the generated efficacy level guarantees moving to a phase III study. In this case, we would hope for a success rate of 13 or more. Given that this is a double blind, randomized, phase II trial with two therapeutic arms, fifty patients were included in each group, i.e., 100 subjects in total.
The analyses of the trial result were performing according to the intention-to- treat principle. Considering that this is a phase II study, the efficacy results were based on the results in the Diamel treatment group.
Qualitative variables summarized in absolute values and percentages. For quantitative variables, the mean, standard error of the mean, and median estimated. The Mantel-Haenszel Chi-square statistical calculation used to compare the two groups (Diamel vs. placebo) in a stratified analysis by smoking habit and pressure index, both factors with a potential confounding effect. Student’s t-test for paired samples used to compare the differences between means of the quantitative variables (before/after) in the Diamel group.
The nonparametric Kaplan-Meier method was used to calculate the conditional probability of epithelialization or healing from time t0 to a given time, as well as the mean and median healing times. For comparison between groups (Placebo vs. Diamel) in relation to this variable, the “failure” curves (1-S) of both groups compared. The log-rank test applied to assess the magnitude of the differences between the curves. 95% confidence interval (CI) of the rates at 12 months was estimated [30,31].
The IBM statistical package used for statistical processing, SPSS. V. 21.0 for Windows.
Ethical considerations: The study approved by the Research and Ethics Committee of the National Institute of Endocrinology and conducted in accordance with the Declaration of Helsinki. Patients participated in the study voluntarily by means of signed informed consent.
Of the 206 patients evaluated, 100 were included and met the selection criteria. The mean age of the patients included was over 60 years. There was a slight predominance of men (59%) and a time of evolution of DM of 18 years or more. The mean insulin dose used was around 30 IU. The explanatory variables showed a similar behavior between the two groups at the beginning of the study (Table 1), except for smoking, which was more frequent in the placebo group (48% versus 20%).
| Variable | Diamel® Group (n=50) No. (%) |
Placebo Group (n=50) No. (%) |
Total |
|---|---|---|---|
| Male | 30 (60.0) | 29 (59.0) | 59 |
| Female | 20 (40.0) | 21 (21.0) | 41 |
| Smoker | 10 (20.0) | 24 (48.0) | 34 |
| Non-smoker | 40 (80.0) | 26 (52.0) | 66 |
| Pressure index | |||
| No arterial disease | 21 (42.0) | 19 (38.0) | 40 |
| Mild arterial disease | 29 (58.0) | 31 (62.0) | 60 |
| Ulcer location | |||
| Toe | 36 (72.0) | 33 (66.0) | 69 |
| Sole and heel | 12 (24.0) | 12 (24.0) | 48 |
| Dorsum | 2 (4.0) | 5 (10.0) | 14 |
| Affected foot | |||
| Left | 29 (58.0) | 23 (46.0) | 52 |
| Right | 21 (42.0) | 27 (54.0) | 48 |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 64.6 (9.4) | 63.5 (10.9) | 64.0 (10.5) |
| Duration of DM (years) | 18.9 (6.1) | 18.8 (5.8) | 18.8 (5.9) |
| Insulin dose (IU/kg) | 30.9 (16.9) | 31.1 (20.3) | 31.0 (18.6) |
| BMI (kg/m2) | 27.6 (4.1) | 28.1 (3.6) | 27.8 (3.8) |
| Systolic blood pressure (mmHg) | 131.6 (21.3) | 132.8 (15.7) | 132.2 (18.5) |
| Diastolic blood pressure (mmHg) | 79.3 (5.7) | 80.7 (8.3) | 79.5 (7.0) |
| Cholesterol (mmol/L) | 5.3 (0.9) | 5.3 (0.6) | 5.3 (0.75) |
| Triglycerides (mmol/L) | 2.3 (0.9) | 2.8 (2.4) | 2.5 (1.8) |
| Creatinine (mmol/L) | 87.9 (20.2) | 87.6 (16.0) | 87.7 (18.1) |
| Glucose (mmol/L) | 10.7 (0.9) | 9.5 (3.7) | 10.1 (2.3) |
| HbA1c (%) | 8.1 (1.6) | 7.9 (1.6) | 8.0 (1.6) |
BMI (body mass index); SD (standard deviation)
Table 1: Characteristics of the groups at the beginning of the study.
As for the location of the ulcers, 52 of the patients had ulcers on their left foot and the other 48 on their right. According to the PI, 60 patients had mild arterial disease, while the other 40 subjects did not present this condition. In 69 patients, the lesion occurred on a toe; in 24 of them, on the sole of the foot and on the heel; and in 9, on the dorsum of the foot (Table 2).
| Adjustment variable | Ratio of those healed between groups | χ² | P | ||
|---|---|---|---|---|---|
| n (Diamel/Placebo) | Diamel® | Placebo | Total | ||
| All subjects (n=50/50) | 86.0% | 68.0% | 77.0% | 4.5737 | 0,032 |
| Smokers (10/24) | 90.0% | 66.7% | 73.5% | 1.9745 | 0,160 |
| Non-smokers (40/26) | 85.0% | 69.2% | 78.8% | 2.3446 | 0,126 |
| Mantel-Haenszel chi-square (1 gl) | 4.1700 | 0.0410 | |||
| No arterial disease (19/21) | 89.5% | 66.7% | 77.5% | 2.9755 | 0,085 |
| Mild arterial disease (31/29) | 83.9% | 69.0% | 76.7% | 1.8609 | 0,173 |
| Mantel-Haenszel chi-square (1 gl) | 4.51 | 0.0338 | |||
Table 2: Analysis of healing and treatment group, adjusted by smoking habit and pressure index.
During follow-up, 6 patients in the placebo group discontinued treatment, 3 of them due to ulcer progression. The remaining 3 discontinued treatment but had healed before month 12. In the Diamel group, there were 4 patients who discontinued treatment: one of them due to lesion progression and the remaining 3 due to voluntary dropout after the lesion had healed.
At the end of the study, 34 (86%) of the patients in the Diamel group achieved total epithelialization of the ulcer (healing) compared to 68% in the placebo group. χ2 = 4.5737; P = 0.032.
A stratified analysis by smoking status showed no statistically significant difference in the proportion of heal patients between treatment groups when the analysis is carried out separately for smokers and non-smokers. However, the adjusted chi-square test showed significant differences between groups with respect to treatment. Mantel-Haenszel chi-square (1 gl) = 4.17; P = 0.0410. Similar results obtained when stratifying and subsequently adjusting by the pressure index for patients with and without arterial disease. Mantel-Haenszel chi-square (1 gl) = 4.51; P = 0.0338.
When comparing time to healing in both groups using the Kaplan-Meier method, 12-month healing rates of 89.2% (95% CI, 77.4-96.5) and 72.4% (95% CI, 58.0-85.1) were observed for the Diamel and Placebo group, respectively. The average healing time was 3 months for the Diamel group and 5 months for the placebo group. The log-rank test showed statistically significant results between the curves: χ2 = 6.9; P = 0.0086 (Figure 1).
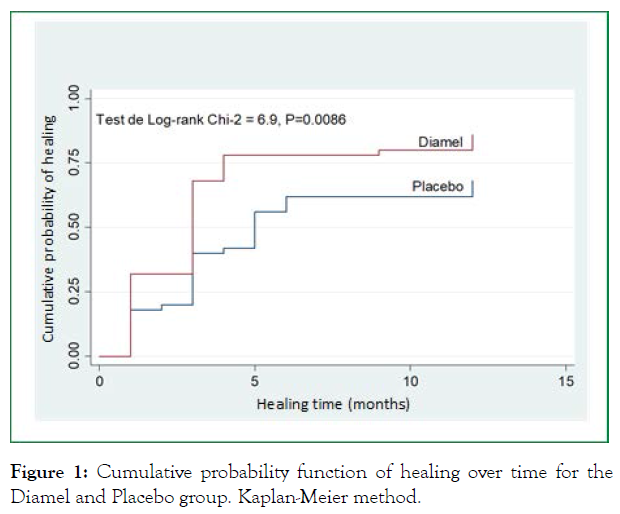
Figure 1: Cumulative probability function of healing over time for the Diamel and Placebo group. Kaplan-Meier method.
Recurrence rates were lower in the Diamel® group (3 patients, 8%) than in the placebo group (8 patients, 16%).
Comparison of blood test values performed before and after treatment in the Diamel group showed a statistically significant decrease in mean fasting glucose and HbA1c levels at the end of the study compared to the time of inclusion (Tables 3 and 4).
| Variable | Diamel® | Placebo | Pr(|T| > |t|) 2 | ||
|---|---|---|---|---|---|
| Before | After | Pr(|T| > |t|) 1 | After | ||
| BMI | 27.5 (4.1) | 23.5 (12.1) | 0,334 | 21.6 (12.4) | 0,439 |
| Cholesterol | 5.3 (0.9) | 5.1 (0.7) | 0,189 | 5.3 (0.6) | 0,375 |
| Triglycerides | 2.3 (0.9) | 2.1 (0.8) | 0,519 | 2.8 (2.4) | 0,069 |
| Creatinine | 88.1 (21.8) | 84.8 (14.7) | 0,183 | 87.2 (11.5) | 0,360 |
| Blood glucose level | 10.7 (3.8) | 8.2 (2.5) | 0,003 | 9.5 (3.7) | 0,064 |
| HbA1c | 8.1 (1.6) | 7.2 (1.28) | 0,000 | 7.9 (1.68) | 0,025 |
1 Student’s t-test for paired samples. 2 t-test for independent samples.
Table 3: Comparison of the mean biochemical variables before and after in the Diamel group and at the end of the study between treatment groups.
| Variable | Diamel® | ||
|---|---|---|---|
| Before | After | Pr(|T| > |t|) 1 | |
| BMI | 27.5 (4.1) | 23.5 (12.1) | 0.334 |
| Cholesterol | 5.3 (0.9) | 5.1 (0.7) | 0.189 |
| Triglycerides | 2.3 (0.9) | 2.1 (0.8) | 0.519 |
| Creatinine | 88.1 (21.8) | 84.8 (14.7) | 0.183 |
| Blood glucose level | 10.7 (3.8) | 8.2 (2.5) | 0.003 |
| HbA1c | 8.1 (1.6) | 7.2 (1.28) | 0.000 |
1 Student’s t-test for paired samples.
Table 4: Comparison of the mean biochemical variables before and after in the Diamel group.
Only one patient experienced heartburn during the course of treatment.
The appearance of DFU is associated with multiple clinical and metabolic risk factors, such as old age, long duration of DM, smoking, and poor metabolic control, among others, factors that also characterized the patients at the beginning of this study [32-42].
Healing is a complex, dynamic, and multifactorial process in which nutrition plays a key role. In a qualitative review, Kulprachakarn [43] emphasizes the role of oral micronutrients and the importance of ingestion of vitamins B, D, E, and C, as well as minerals and trace elements such as zinc, magnesium, and iron, among others, in tissue repair both in vitro and in vivo.
At the end of the study, more than 80% of the patients in the Diamel group achieved total epithelialization (healing) of the ulcer, exceeding the expected 30%. This variable considered the main variable of response to treatment.
In the present investigation, ulcer healing occurred in a shorter period in patients who took Diamel® than in those in the Placebo group, suggesting that Diamel has added benefits over the usual treatment of DFU. This product contains trace elements, amino acids, vitamins, and lettuce and blueberry extracts, components that can act as antioxidants, thus decreasing the formation of free radicals and accelerating the healing process [22,25,26]. Blueberry contains amino acids and tannins that improve localized microcirculation in and around the ulcer. A recent investigation concluded that daily consumption of 22 g of freeze-dried blueberries was beneficial to cardiometabolic function in men with DM2 due to improved glycemic control and dyslipidemia [44].
Several studies prove the efficacy of various products used in DFU healing. Such is the case of the study by Franco [45] with snail slime, which is used topically; and that of Buzzi [46], which tested the efficacy and safety of Calendula officinalis extract in patients with uncomplicated DFU and demonstrated its superiority over placebo in terms of healing time, recovered tissue area, and superinfection.
In the present trial, a synergistic effect on patient healing achieved, which occurred, in our opinion, for several reasons. On the one hand, the integral treatment based on conventional therapy plus the product under study favored a substantial improvement in metabolic control, which was useful for better DFU healing. The good glycemic control achieved also promotes rapid and efficient healing of DFU [41,46,47]. It has been demonstrated that certain nutritional supplements contain polyphenols that act on oxidative stress and favor a better evolution of many DM complications [17,18,22]. Moreover, the supply of B vitamins blocks the damaging effect of free radicals and provides a favorable microenvironment for healing [43,46-50].
The beneficial effect of an educational intervention in reducing fasting plasma glucose and the importance of structuring educational programs to reduce the occurrence and progression of complications have also been demonstrated [47]. Both groups received guidance on a healthy lifestyle, as is usual in the intervention strategies of the health system in Cuba.
At the end of the study, the Diamel® group showed a decrease in glycosylated hemoglobin values close to optimal control, as well as in fasting glycemia. Other research found similar result when using Diamel® in adults with metabolic syndrome [25] and in women with polycystic ovary syndrome [26]. A controlled study in 30 patients with DM2 using Diamel® + glibenclamide versus glibenclamide alone showed that changes in metabolic control were significantly better in the Diamel® group, with a decrease in the daily dose of glibenclamide at six months [21].
The effect of Diamel® in the present investigation was not modified by whether people smoked or whether they had mild arterial disease. However, all care programs for patients with diabetes include the recommendation to stop this toxic habit, [47,51] which was achieved in most patients from the beginning of treatment. However, one limitation of the study was that no detailed record was made of the smoking habit in terms of frequency, time of exposure, and intensity.
The large ratios of patients whom total epithelialization of the lesion achieved, in a relatively short period and under metabolic control, as well as the absence of significant adverse events, support the fact that the nutritional supplement Diamel® showed a sufficient degree of efficacy to propose large-scale studies. This means it could become a useful alternative as a adjunct to the treatment of DFU in patients with type 2 DM treated with insulin.
Conflict of interest: The authors claim that there is no conflict of interest. Funding statement: This study was partly funded by Catalysis, S.L., who provided both products (Diamel® and placebo) used for the trial.
Authors’ contribution
Marelys Yanes-Quesada: Implementation, Preparation of the final report, Discussion of the results, presentation of results in meetings with Catalysis and congresses
Daysi Navarro-Despaigne: Participation in the preparation of the final report, Discussion of the results, presentation of results in meetings with Catalysis and congresses
Eduardo Cabrera-Rode: Contribution to the discussion of the results, search for updated bibliography.
Obdulio Hernández-González. Cooperation in statistical analysis
Juan J Lence-Anta: Preparation of the statistical analysis proposal, Preparation of the randomization
Roberto Pena-Gener: Angiology evaluation of patients with diabetic foot ulcers
Citation: Yanes-Quesada M, Navarro-Despaigne D, Cabrera-Rode E, Lence-Anta JJ, Gonzalez-Hernández O, Peña-Gener R, Sanz-Navares E (2021) Effectiveness of Diamel® In the Treatment of Diabetic Foot Ulcers: A Randomized and Placebo-Controlled Phase II Clinical Trial. J Diabetes Metab. 12:891.
Received: 31-Jul-2021 Published: 26-Aug-2021, DOI: 10.35248/2155-6156.21.12.891
Copyright: © 2021 Yanes-Quesada M, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.