Research Article - (2020) Volume 11, Issue 6
Background: Foods rich in protein, dietary fibre and low in glycaemic index could potentially improve glycaemic control and help in management of type 2 diabetes mellitus (T2DM) in overweight/obese participants. In this study, the effect of high-protein high-fibre (HPHF) nutritional supplement in addition to standard medical care for glycaemic control was evaluated.
Methods: In this open-label, parallel-arm, randomised study, 100 overweight/obese participants with T2DM (aged 30-65 years) were randomised (1:1) to either intervention group (25 g HPHF nutritional supplement [twice daily]+standard care of T2DM) or a control group (standard care of T2DM), for 6 months. Primary endpoint included change from baseline in 24��?hour glycaemic response at weeks 12 and 24.
Results: Of 320 participants screened, 100 participants were randomised to either the intervention group (n=50) or the control group (n=50). In the intervention group; the mean (SE) daily incremental area under curve (IAUC) from baseline to week 24 was significantly lower ��?23.0 mg-15min/dL (57.2) compared to control group 168.0 mg-15min/ dL (39.0), p=0.008. The intervention group showed significant reduction in glycosylated haemoglobin (HbA1c) (p=0.03) and fasting blood sugar (p=0.01) level at the end of week 24 compared to the control group.
Conclusions: Twice-daily consumption of HPHF nutritional supplement (25 g each) significantly improved glycaemic control, reduced the average 24-hour glycaemic response and postprandial glucose spikes. Inclusion of HPHF supplement would be a useful effective aid to glycaemic control in overweight/obese participants with T2DM.
Dietary intervention; Glycaemic control; High-fibre; High-protein; Lifestyle; Nutritional supplement; Obesity;ype 2 diabetes
Type 2 diabetes mellitus (T2DM) has reached epidemic proportions worldwide with India having the second largest number of people with diabetes (77.0 million as of 2019) [1]. The Chennai Urban Rural Epidemiology Study (CURES) demonstrated higher incidence of diabetes (29.5 per 1000 person-years) in individuals with advancing age, family history of diabetes, higher 2-hours plasma glucose and glycosylated haemoglobin (HbA1C), low highdensity lipoprotein (HDL) cholesterol, and physical inactivity [2,3]. Rapid transition of individuals with normal glucose tolerance to diabetes (19.4%) and prediabetes (25.7%) underscores importance of public health interventions in preventing T2DM [3,4]. Overweight and obesity are major risk factors in the development of T2DM with reported prevalence of diabetes being 20.2% in obese and 15.5% in overweight individuals [5-7]. Rapid change in food habits, unhealthy diet (consumption of high refined carbohydrate, lower proportion of proteins and fibres) and low physical activity are the major factors associated with overweight, obesity and associated T2DM [8-11].
Optimal glycaemic control in individuals with diabetes is best achieved by medical nutrition therapy. However, there are many difficulties in initiating and maintaining it over the long term. Dietary consistency in individuals with diabetes can be ensured by prescribing Diabetes Specific Nutrition Supplement (DSNS) along with drug treatment to prevent striking bloodglucose excursions [12]. Inclusion of DSNS showed significant reductions in peak blood glucose concentration, postprandial glucose (PPG), glucose area under the curve and HbA1C [12,13]. Hence, studies have mainly emphasized on the importance of highfibre, lowcarbohydrate, low-fat and energy restricted high-protein diet in sustaining weight loss and minimizing the risk of T2DM or cardiovascular disease (CVD) in overweight/obese individuals [14].
The American Diabetes Association (ADA) also recommends intake of low glycaemic index (GI) food along with adequate protein and fibre for improvement of lipid profile and prevention of CVDs in overweight/obese individuals with T2DM [15,16]. Postprandial or acute hyperglycaemia is a major rate-limiting factor for achieving optimal glycaemic control and increases risk of CVD [17-19]. Food constituents like fibre tend to bring down glycaemic response and exhibit beneficial outcomes by reducing HbA1c and PPG levels [15]. Apart from medical interventions, a balanced low GI diet rich in proteins and fibre potentially remains a key strategy for improvement of glycaemic control and optimal management of T2DM and its complications in overweight and obese individuals. A plantbased high-protein and high-fibre (HPHF) dietary supplement has been shown to have low GI in overweight/obese adults [20].
Thus, this study aimed to evaluate the effect of HPHF nutritional product on glycaemic markers-fasting blood glucose, HbA1c and average 24-hour glycaemic response at weeks 12 and 24 in overweight/obese adults with T2DM.
Study participants
Participants in this parallel-arm, open-label, randomised controlled trial were identified from the medical records of a tertiary care centre for diabetes in Chennai, India, based on pre specified inclusion and exclusion criteria. Inclusion criteria were: age 30-65 years of either gender, diagnosed with T2DM of at least 1 year duration, treated with stable doses of oral antidiabetic drugs for at least 3 months before screening, HbA1c from 7.0% to 12.0%, and body mass index (BMI) of ≥ 23 kg/m2 and <30 kg/m2 (Asian Indian cut off for overweight and obesity) [21].
Participants with T2DM who were on insulin injections or on unstable doses of oral hypoglycaemic agents (OHA) in the last 3 months were excluded from the study. Participants who had a history of acute infections in the last 1 month, respiratory disorders, eating disorders or lactose intolerance, hypoglycaemia in last 3 months, cancer or malignancy, heart attack, or stroke in the past 1 year were also excluded from the study. Additionally, participants who were on herbal or ayurvedic or traditional medicines that could affect blood glucose were excluded from the study. Other exclusion criteria were pregnant and lactating women, individuals who were planning to relocate within 2 years after study initiation or who had plans of longer duration of travel out of town.
Eligible participants identified via this medical record review (n=320) were then contacted and briefed by research dieticians about the study and its objectives (Figure 1). Individuals expressing an interest in participating were given 25 g of HPHF powder twice a day for one week free of cost to assess their willingness to consume and comply with the study criteria. Individuals who completed the run-in period of 1 week and expressed their willingness to comply and take part in the study were randomised using computer-generated random numbers to either the intervention (n=50) group or the control (n=50) group and completed the baseline visit one week after the run-in period.
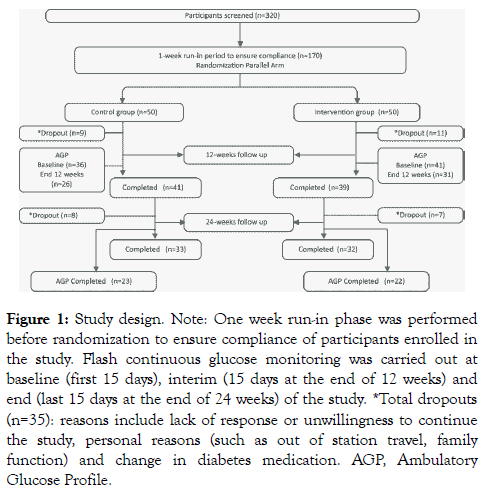
Figure 1: Study design. Note: One week run-in phase was performed before randomization to ensure compliance of participants enrolled in the study. Flash continuous glucose monitoring was carried out at baseline (first 15 days), interim (15 days at the end of 12 weeks) and end (last 15 days at the end of 24 weeks) of the study. *Total dropouts (n=35): reasons include lack of response or unwillingness to continue the study, personal reasons (such as out of station travel, family function) and change in diabetes medication. AGP, Ambulatory Glucose Profile.
The study protocol and informed consent form were reviewed and approved by the Independent Ethics Committee and the Institutional Review Board and registered in the clinical trial registry of India CTRI/2018/04/012979 [dated 03/04/2018]. The study was conducted in accordance with the ethical principles of Declaration of Helsinki and per the International Council for Harmonization and Good Clinical Practice guidelines. All participants provided written informed consent prior to study enrolment.
Dietary intervention
Participants in the intervention group were instructed to consume 25 g HPHF nutritional supplement (one with breakfast and one in the evening) for 24 weeks along with standard care of diabetes. The participants were asked to mix 25 g HPHF nutritional supplement (provided as a sachet) with ~200 mL water and consume the mixture without any leftover. For both the groups, all dietary advice was individualized and provided by dieticians, as is standard practice for the tertiary care centre for diabetes in Chennai, India, where this study was conducted.
Participant compliance was assessed using the dietary 24-hour recall collected by trained dieticians in a face-to-face interview. EpiNu nutrient database (Madras Diabetes Research Foundation, India) was used to assess the food and nutrient intake (mainly macronutrients) from the 24-hour dietary recalls. The average of 5 recalls collected during the 24 week intervention was compared to the baseline (3 days dietary recall at baseline) in order to improve the precision and accuracy of the estimates of dietary intake during the intervention period.
Outcome assessments
Anthropometry: Anthropometric measurements including body weight (kg) (electronic OMRON machine; 171 Omron HBF 212, Tokyo, Japan), height (cm), and waist circumference (cm) were measured at baseline, monthly once and at the end of study according to the standard protocols. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2).
Blood Pressure: Blood pressure was assessed twice on each occasion (baseline, every month and end of study) at 5-minute intervals using an electronic OMRON machine (Omron HEM 7120, Tokyo, Japan). Participants were seated comfortably with back straight and feet flat on the floor and the average of the two readings was taken after 10 mins of rest.
Ambulatory Glucose Profile (AGP) Assessment: A Free Style Libre Pro™ (Abbott Diabetes Care, India) Flash Glucose Monitoring (FGP) System consisting of a reader and a sensor (inserted in the back of the upper arm) was used to analyse the interstitial fluid glucose levels for AGP assessment in consenting participants. The interstitial fluid glucose levels were assessed at the beginning and at the end of 2 weeks of the study as a measure of participant compliance. Ambulatory glucose monitoring was done at baseline (first 15 days), interim (for 15 days at the end of 12 weeks) and at the end (last 15 days at the end of 24 weeks).
Biochemical: Blood samples (5 mL) were collected for assessment of fasting blood glucose, HbA1c, lipid profile at baseline, and at the end of 12 and 24 weeks. Fasting (≥ 8 hours) venous blood samples were collected into tubes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. Plasma glucose was estimated using the glucose oxidaseperoxidase method (Roche Diagnostics, Basel, Switzerland). The HbA1c was estimated by high-pressure liquid chromatography using the Variant machine (Bio-Rad, Hercules, CA). All the laboratory assessments were done in a National Accreditation Board for Testing and Calibration Laboratories certified laboratory.
Adverse events if any were assessed; recorded and appropriate medical intervention was given.
Statistical analysis
Statistical analysis was performed using SAS 9.2 version. Baseline demographics and clinical characteristics were estimated using independent t-tests for continuous variables and Pearson chi-square test for categorical variables. Generalized linear Model (GLM) was used to assess the main effects of changes in anthropometric, blood parameters and nutrient intake at different time points (baseline vs end of 12 weeks and end of 24 weeks) and covariate (diabetic medication count/day) adjusted as an effect of interaction between and within the groups and reported as least square mean and standard error. Change from baseline over 12 and 24 weeks were measured as positive incremental area under the cure (IAUC) to assess the effect of HPHF supplement on glycaemic response (interstitial glucose concentration) through AGP. The significance was further tested with independent ‘t’ test. Change in interstitial glucose over 13 days (24 hours every day) at baseline versus at the end of 12 and 24 weeks with another 13 days of IAUC at each time point was considered for this analysis. To determine the daily baseline value for each participant, we used the average of each day ’s fasting 2 hour (6 to 8 am) interstitial glucose concentrations. The daily IAUC for each participant was then calculated from the remaining 22hour readings of AGP data. The daily positive area under the curve (AUC) was the sum of all observed 15-min AGP measurement standardized to the number of measurements available. Statistical significance was set at p<0.05 and data was expressed as mean and standard error.
Participant disposition and baseline characteristics
Of 320 participants screened, 100 overweight/obese participants with T2DM were enrolled and randomised to the intervention group (HPHF nutritional supplement daily for 24 weeks with standard care of T2DM) and control group (standard-of-care) (Figure 1). The reasons for screen failure were participants not meeting the inclusion criteria [50% not meeting the HbA1c criteria, or due to other reasons (30%) which includes age, taking insulin therapy, having diabetic complications, currently on medications for heart disease] and not willing to participate in the study (20%). At the end of 24 weeks, 18 participants in the intervention group and 17 in the control group dropped out of the study due to reasons such as lack of response from the participants even after repeated attempts or unwillingness to continue the study, personal reasons including out of station travel, family function and change in diabetes medication in the control group. There were no side effects of the nutritional intervention. The mean age of participants included in the study was ~51 (8.0) years. Both the control and intervention groups were homogeneous and there was no major significant difference in the baseline characteristics including anthropometric, blood pressure, biochemical and dietary macronutrients and energy measurements between the groups (Table 1).
Table 1: Demographic and baseline characteristics of the study participants (per protocol).
| Variables | Control Group (n=41) | Intervention Group* (n= 39) | p value†, ‡ |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 51.0 ± 7.9 | 51.2 ± 8.5 | 0.92 |
| Men, n (%) | 17.0 (41.5) | 23.0 (59.0) | 0.11 |
| Height (cm) | 157.7 ± 10.7 | 161.3 ± 8.4 | 0.11 |
| Weight (kg) | 72.8 ± 14.1 | 73.0 ± 14.6 | 0.95 |
| BMI (kg/m2) | 29.3 ± 5.4 | 27.9 ± 4.5 | 0.21 |
| WC (cm) | 97.7 ± 12.2 | 96.0 ± 10.9 | 0.52 |
| SBP (mmHg) | 126.3 ± 17.5 | 125.7 ± 16.1 | 0.87 |
| DBP (mmHg) | 85.2 ± 10.7 | 82.3 ± 7.7 | 0.17 |
| FBS (mg/dL) | 160.5 ± 54.1 | 172.6 ± 50.7 | 0.3 |
| HbA1c (%) | 8.2 ± 1.3 | 8.8 ± 1.3 | 0.06 |
| Diabetes medication (count) | 2.1 ± 1.0 | 2.6 ± 1.0 | 0.05 |
| Duration of diabetes (years) | 5.3 ± 2.8 | 5.0 ± 2.7 | 0.61 |
| Energy Kcal/day | 1567 ± 339 | 1428 ± 328 | 0.06 |
| Carbohydrates %E§ | 58.0 ± 5.9 | 58.4 ± 5.4 | 0.77 |
| Protein %E§ | 12.2 ± 1.2 | 12.3 ± 1.5 | 0.79 |
| Fat %E§ | 24.6 ± 4.7 | 24.3 ± 5.0 | 0.78 |
| Total dietary fibre g/d§ | 25.2 ± 5.3 | 26.3 ± 6.2 | 0.36 |
Note: *High-Protein High-Fibre 2 sachet (intervention) for 24 weeks. †Significance tested using Independent-t test. ‡Significance test using chi-square test. §Energy adjusted nutrients. BMI, Body Mass Index; DBP, Diastolic blood pressure; FBS, Fasting blood sugar; HbA1c, glycosylated haemoglobin; SBP-Systolic blood pressure, WC-Waist Circumference.
Glycaemic response study
The mean (SE) change in IAUC from baseline to week 12 was significantly lower in intervention group [-30.0 mg-15min/dL (54.3)] compared to control group [112.3 mg15min/dL (41.0), p=0.04] (Figure 2). Similarly, at the end of 24 weeks, the mean (SE) change in IAUC for the intervention group was significantly lower [23.0 mg15min/dL (57.2)] compared to control group [168.0 mg-15min/dL (39.0), p=0.008] (Figure 3). At week 12, there were no significant changes in HbA1c and fasting blood sugar level (FBS) in both the groups. However, the intervention group showed significant improvement in protein (p=0.001) and total dietary fibre intake (p=0.001) (Table 2).
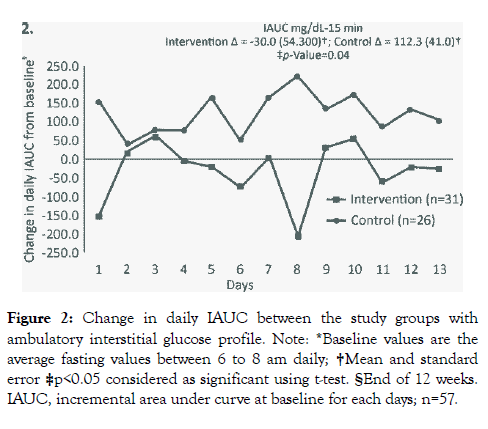
Figure 2: Change in daily IAUC between the study groups with ambulatory interstitial glucose profile. Note: *Baseline values are the average fasting values between 6 to 8 am daily; †Mean and standard error ‡p<0.05 considered as significant using t-test. §End of 12 weeks. IAUC, incremental area under curve at baseline for each days; n=57.
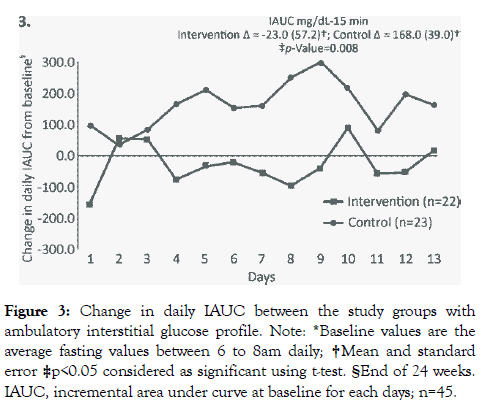
Figure 3: Change in daily IAUC between the study groups with ambulatory interstitial glucose profile. Note: *Baseline values are the average fasting values between 6 to 8am daily; †Mean and standard error ‡p<0.05 considered as significant using t-test. §End of 24 weeks. IAUC, incremental area under curve at baseline for each days; n=45.
Table 2: Change in anthropometric, biochemical parameters and nutrients intake between the groups: 12 weeks.
| Variables | Control group (n=41) | Intervention group* (n=39) | Difference between groups p value‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline LSM (SE)† |
12 week LSM (SE)† |
Change LSM (SE)† |
p value‡ | Baseline LSM (SE)† |
12 week LSM (SE)† |
Change LSM (SE)† |
p value‡ | ||
| Weight (kg) | 72.9 (2.0) | 72.6 (2.0) | -0.3 (0.2) | 0.76 | 73.0 (3.0) | 72.0 (3.0) | -1.0 (0.3) | 0.80 | 0.56 |
| BMI (kg/m2) | 29.4 (0.8) | 29.2 (0.8) | -0.2 (0.1) | 0.84 | 27.8 (1.0) | 27.6 (1.0) | -0.2 (0.1) | 0.78 | 0.91 |
| WC (cm) | 98.0 (2.0) | 98.3 (2.0) | 0.3 (0.4) | 0.98 | 96.0 (2.0) | 96.0 (2.0) | 0.0 (0.4) | 0.90 | 0.65 |
| SBP (mmHg) | 127 (3) | 127 (3) | -0.1 (2) | 0.73 | 126 (3) | 122 (3) | -4 (2) | 0.23 | 0.13 |
| DBP (mmHg) | 85 (2) | 84 (2) | -1 (2) | 0.83 | 83 (2) | 81 (2) | -1 (2) | 0.67 | 0.93 |
| FBS (mg/dL) | 156 (9) | 168 (11) | 12 (10) | 0.47 | 179 (9) | 179 (12) | -0.3 (11) | 0.90 | 0.40 |
| HbA1c (%) | 8.1 (0.2) | 8.3 (0.3) | 0.2 (0.2) | 0.70 | 9.0 (0.2) | 9.0 (0.3) | 0.0 (0.2) | 0.76 | 0.47 |
| Diabetes medication (count) | 2.4 (0.0) | 2.3 (0.1) | -0.1 (0.1) | 0.64 | 2.4 (0.0) | 2.3 (0.1) | -0.1 (0.1) | 0.25 | 0.92 |
| Energy Kcal/day | 1572 (56) | 1570 (46) | -2 (44) | 0.88 | 1433 (60) | 1485 (49) | 52 (48) | 0.30 | 0.41 |
| Carbohydrates %E | 58.0 (1.0) | 56.0 (1.0) | -2.0 (1.0) | 0.06 | 58.0 (1.0) | 55.0 (1.0) | -3.0 (1.0) | 0.02 | 0.56 |
| Protein %E§ | 12.2 (0.2) | 12.3 (0.2) | 0.1 (0.3) | 0.94 | 12.0 (0.2) | 14.0 (0.2) | 2.0 (0.3) | 0.001 | 0.001 |
| Fat %E§ | 25.0 (1.0) | 26.0 (1.0) | 1.0 (1.0) | 0.30 | 25.0 (1.0) | 26.0 (1.0) | 1.0 (1.0) | 0.40 | 0.73 |
| Total Dietary fibre %E§ | 25.0 (1.0) | 24.0 (1.0) | -2.0 (1.0) | 0.13 | 27.0 (1.0) | 30.0 (1.0) | 3.0 (1.0) | 0.004 | 0.001 |
Note: *High Protein High Fibre 2 sachet (Intervention) for 24 weeks. †Least square Mean and standard error. ‡p<0.05 considered as significant using generalized linear Model. §Energy adjusted nutrients. Mean-Outcome adjusted for Diabetes medication count per day. BMI, Body Mass Index; DBP, Diastolic blood pressure; FBS, Fasting blood sugar; HbA1c, glycosylated haemoglobin; SBP-Systolic blood pressure, WC-Waist Circumference.
Significant reductions were noted in the intervention group for HbA1c [-0.3 (0.3), p=0.03] and FBS [-16 (12), p=0.01] level at the end of 24 weeks compared to the control group (Table 3). A significant increase in protein (p=0.001) and total dietary fibre (p=0.003) was observed in the intervention group at the end of 24 weeks. However, no such change was observed in control group (Table 3).
Table 3: Change in anthropometric, biochemical parameters and nutrients intake between the groups: 24 weeks.
| Variables | Control group (n=33) | Intervention group* (n=32) | Difference between groups p value‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline LSM (SE)† |
12 week LSM (SE)† |
Change LSM (SE)† |
p value‡ | Baseline LSM (SE)† |
12 week LSM (SE)† |
Change LSM (SE)† |
p value‡ | ||
| Weight (kg) | 74.0 (3.0) | 73.0 (2.0) | -1.0 (0.4) | 0.92 | 72.0 (3.0) | 71.7 (3.0) | -0.3 (0.3) | 0. 71 | 0.55 |
| BMI (kg/m2) | 29.4 (0.9) | 29.4 (0.9) | 0.0 (0.3) | 0.86 | 27.9 (0.9) | 27.8 (0.9) | -0.03 (0.3) | 0.76 | 0.86 |
| WC (cm) | 98.0 (2.0) | 97.0 (2.0) | -1.0 (1.0) | 0.85 | 95.5 (2.2) | 95.3 (2.1) | -0.2 (0.6) | 0.70 | 0.69 |
| SBP (mmHg) | 127 (3) | 121 (3) | -6 (3) | 0.18 | 125 (3) | 121 (3) | -4 (3) | 0.15 | 0.59 |
| DBP (mmHg) | 85 (2) | 86 (2) | 0.2 (2) | 0.62 | 82 (2) | 82 (2) | 0.3 (2) | 0.75 | 1.0 |
| FBS (mg/dL) | 155 (10) | 180 (13) | 25 (11) | 0.30 | 180 (10) | 164 (14) | -16 (12) | 0.60 | 0.01 |
| HbA1c (%) | 8.0 (0.2) | 8.5 (0.3) | 0.5 (0.3) | 0.44 | 9.0 (0.2) | 8.6 (0.3) | -0.3 (0.3) | 0.66 | 0.03 |
| Diabetes medication (count) | 2.5 (0.0) | 2.6 (0.0) | 0.1 (0.0) | 0.37 | 2.5 (0.0) | 2.3 (0.0) | -0.2 (0.0) | 0.14 | 0.10 |
| Energy Kcal/day | 1648 (56) | 1674 (53) | 26 (46) | 0.26 | 1435 (58) | 1544 (54) | 109 (48) | 0.07 | 0.22 |
| Carbohydrates %E | 59.0 (1.0) | 55.0 (1.0) | -4.0 (1.0) | 0.01 | 58.0 (1.0) | 54.0 (1.0) | -4.0 (1.0) | 0.002 | 0.92 |
| Protein %E§ | 12.0 (0.3) | 12.0 (0.5) | -0.4 (0.5) | 0.24 | 12.0 (0.2) | 14.0 (0.4) | 2.0 (1.0) | 0.001 | 0.001 |
| Fat %E§ | 24.0 (1.0) | 26.0 (1.0) | 2.0 (1.0) | 0.30 | 25.0 (1.0) | 28.0 (1.0) | 3.0 (1.0) | 0.06 | 0.32 |
| Total Dietary fibre %E§ | 26.0 (1.0) | 24.0 (1.0) | -2.0 (1.0) | 0.34 | 27.0 (1.0) | 30.0 (1.0) | 3.0 (1.0) | 0.007 | 0.003 |
Note: *High Protein High Fibre 2 sachet (Intervention) for 24 weeks. †Least square Mean and standard error. ‡p<0.05 considered as significant using generalized linear Model. §Energy adjusted nutrients. Mean-Outcome adjusted for Diabetes medication count per day. BMI, Body Mass Index; DBP, Diastolic blood pressure; FBS, Fasting blood sugar; HbA1c, glycosylated haemoglobin; SBP-Systolic blood pressure, WC-Waist Circumference.
No significant changes were observed for body weight, systolic blood pressure (SBP) or diastolic BP (DBP) at the end of weeks 12 and 24 for both the groups (Table 2 and 3).
The present study evaluated the effect of a HPHF nutritional supplement on glycaemic response in overweight/obese individuals with T2DM in India. The results from 12 and 24 weeks flash glucose monitoring demonstrated significant improvement in glycaemic parameters following dietary intervention with HPHF nutritional supplement when compared to control group. These results are consistent with the previous trials conducted using similar diabetes-specific formulas. Chee et al. demonstrated that the intervention group taking DSNS along with standard-of-care showed significant improvement (-1.1 ± 0.1%, p<0.001) in HbA1c at week 24 compared to the usual care in overweight/obese, T2DM participants [22].
Several studies have reported Indian population to be protein and fibre deficient. A recent study concluded that about 58% participants consumed less than the recommended median dietary fibre intake of 29 g/day, whereas a very high content (about >50 g/day) is required to improve HbA1c in individuals with diabetes [23,24]. Similarly, dietary protein intake (49 g/day) was less than the recommended intake of 56-60 g/day [25]. Considering the consequences of low fibre and low protein dietary intake, we carefully designed our nutritional supplement to be high in proteins, (approximately 30%) (plant proteins like soy bean and bengal gram) and fibre (12%), with low GI (approximately 27). Use of this supplement in overweight and obese participants with T2DM showed significant improvement in glycaemic parameters when included with standard care as compared to the standard-of-care alone. Our results are consistent with those of a similarly designed 24-week study conducted in Chinese participants, receiving structured intervention program that includes DSNS [26]. A study showed PPG was significantly lower after consumption of DSNS when compared to oatmeal [27]. A similarly designed pilot study conducted in overweight and obese Indian adults with T2DM also demonstrated that the intervention group receiving highfibre content through the DSNS showed improved glycaemic control and reduced glycaemic response [12].
Studies have reported indigestible carbohydrates to reduce PPG for the subsequent meal [28]. The inclusion of fibre in food could also additionally help in reducing PPG of a meal by slowing its digestion rate or by reducing glucose absorption from the intestine [29,30]. It has been observed that low GI evening meal could significantly lower the PPG to the next morning when compared to high GI evening meal (6.36 vs. 6.91, respectively; p <0.05) [31]. The positive impact of low GI food in lowering glycaemic response throughout night and extended effect after consumption of lower GI breakfast in the morning has been shown in a randomised double-blinded study in Indian population. It was also shown that pre-consumption of low GI diet could lower glycaemic response even with a standard dinner meal [32]. Overall, evidence suggests breakfasts with limited rapidly available carbohydrate, rich in fibre and protein content with low GI have beneficial effects on glycaemic parameters [28]. Based on the positive impact of one meal on glycaemic response of subsequent meals, we designed our study to provide HPHF nutritional supplement twice daily i.e., with breakfast and as an evening meal to maintain desirable glucose levels over 24 hours. It is noteworthy that the results confirmed significantly low average 24-hour daily IAUC (p=0.04 at week 12 and p=0.008 at week 24) in participants consuming HPHF supplement compared to control group. Similarly, the continuous glucose monitoring (CGM) study of brown rice also exhibited significant lowering of 24-hour glycaemic response(IAUC=34.7 mg*5 min/dL) compared to white rice (58.4 mg*5 min/dL) due to its high fibre content and lower GI [33].
Further, the increase in PPG and impaired glucose tolerance has a direct effect on diabetes comorbidities most importantly coronary heart diseases [27]. Interestingly, a cross-sectional study of participants with coronary heart disease with normal glucose tolerance had a linear correlation of glycaemic surge/ postprandial hyperglycaemia with cardiovascular risks [17]. Most of the cardiovascular risk factors have direct association with acute increase of glycaemia in individuals with diabetes [18]. Therefore, PPG surge has to be prevented to minimise the cardiovascular complications in individuals with diabetes. An economic analysis also confirmed the utilization of DSNS to be cost-effective strategy as the improvement in glycaemic parameters can help decrease the severity and incidence of diabetic comorbidities and thus reduce cost of care per quality of life year gained [34].
Our study design was strengthened with use of standardized and validated flash continuous glucose monitoring method that recorded interstitial glucose levels every 5 minutes providing a comprehensive picture of blood glucose changes compared to other conventional glucose monitoring techniques [35]. Hence, the study could provide robust data to support the clinical benefits of HPHF nutritional supplement in overweight/obese participants with T2DM. The intervention duration of 24 weeks is also one of the strengths of the study. Such long-term studies on individuals with diabetes are very rare. The primary limitations of this study are that the total number of participants studied for continuous glucose monitoring was relatively small.
Our findings suggest that twice daily administration of HPHF nutritional supplement was helpful in improving glycaemic response in Indian overweight and obese adults with T2DM, as compared to standard-of-care alone. Individuals with diabetes need to be educated to increase awareness about the importance of diet and to increase longterm adherence to prevent diabetes complications. Further studies are necessary to more fully characterize the clinical profile of HPHF supplement.
It can be concluded from the intervention trial that inclusion of diabetes specific nutritional supplement along with medication was helpful in improving the glycaemic control including HbA1c and fasting blood glucose level in Indian overweight and obese adults with T2DM, as compared to standard medical care alone. This supplement could be a useful aid to improve the protein and dietary fibre intake in the diets of people with T2DM.
We thank Preethi Bheereddy M.S (Pharm) and Nigar Malik (B. Pharm), both from SIRO ClinPharm (funded by DRL) for writing assistance and editorial support.
All authors participated in the original design of the studies, supervising recruitment and monitoring of data quality, and contributed to the data interpretation, development and review of this manuscript and confirm that they have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors. All authors had access to the study data, provided direction and comments on the manuscript, made the final decision about where to publish these data and approved submission to this journal.
This research was funded by Dr. Reddy’s Laboratories Ltd.
None to report.
Citation: Bhoite R, Chandrasekaran A, Aacharya S, Mane A, Mehta S, Kale RM, et al. (2020) Effect of High-Protein High-Fibre Supplement on Glycaemic Control in Overweight and Obese Indian Adults with Type 2 Diabetes Mellitus: A 24-Week, Randomized, Controlled Trial. J Diabetes Metab 11:846. doi: 10.35248/2155-6156.20.11.846
Received: 01-May-2020 Published: 29-May-2020, DOI: 10.35248/2155-6156.20.11.846
Copyright: © 2020 Bhoite R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.