Research Article - (2016) Volume 4, Issue 2
The influence of temperature and time of heating on the production and composition of organosulfur compounds (OSC) in Allium sativum were investigated in a two-level design with two factors. The organosulfur compounds were identified by Headspace-GC-MS. It was established that temperature plays an important role in the regulation of the production of monosulfides and trisulfides. The effect of both negative and positive temperatures on the production and decomposition of OSC were showed. Although, monosulfides and trisulfides were totally absent at the beginning of incubation, several mono- and trisulfides were detected in high proportions at 120°C, during the 30 minutes incubation period. It appears that high temperature reduces the amount of disulfides, which are converted to monosulfides and trisulfides.
Keywords: Allium sativum; Organosulfur compounds; Factorial design; Headspace-GC-MS
Allium species, especially garlic (Allium sativum) have been cultivated not only as spice but also as medicinal plants all over the world. Garlic (Allium sativum) is one of the earliest known medicinal plants. Its cloves had been used as a cure for many diseases in ancient Egypt and are mentioned in the Ebers Papyrus [1]. In this Codex of Ebers, 22 formulas of garlic were specified for the treatment of various disorders including heart problems, headache, bites, parasites and even tumors [2]. These considerations make the clinical exploitation of garlic derivatives/extracts an attractive and favorable strategy in therapeutic uses. As a result, many epidemiological studies have been conducted to scientifically validate the multi-beneficial effects of garlic on health [3]. The health beneficial effects of garlic and other Allium species have made the family Alliaceae an extremely promising research topic. Recently, studies were published on the potential medicinal uses of garlic by Pedraza-Chaverri et al. [4], Banerjee and Maulik [5] and Abdel-Daim et al. [1,6]. Special importance has been attributed to regular garlic consumption, especially in the prevention of cardiovascular diseases [7,8]. Moreover, cholesterol lowering, hypoglycemic, immune-stimulatory, anti-microbial and even anti-cancer properties have been reported for garlic compounds [9-13]. Epidemiological studies clearly show the correlation between moderate garlic intake and a low cancer incidence with no health risk to consumers [14,15]. A case-control study has been performed on different geographical areas located in different countries (China [16]; Japan [17]; Italy [18]; Netherlands [19] and Venezuela [20]). This beneficial effect of garlic is attributed to their bioactive compounds such as diallyl sulphide (DAS) and other organosulfur compounds (OSC), which have the ability to prevent cancer by acting as efficient free radical scavengers [1].
Ninety-five percent of the sulfur compounds in intact garlic cloves have been found in two chemical classes in similar abundances: the γ-glutamyl-S-alkylcysteines and the S-alkylcysteine sulfoxides (alliin), which represent most abundant sulfur compound in garlic [21].
In fresh garlic alliin is present at 10 mg/g, while in dried garlic its level is 30 mg/g [2]. When garlic cloves are cut, crushed, or chopped (even when the powder of dried cloves becomes wet in a non-acid solution), the odorless cysteine sulfoxides are rapidly converted into a new class of compounds, the thiosulfinates, which are responsible for the odor of freshly chopped garlic. In fact, the odourless compound alliin is converted to allicin, via allinase enzyme. Allicin is the main biologically active component of garlic clove extracts representing about 70% of all thiosulfinates formed in crushed garlic and gives the garlic its characteristic smell [1].
The composition of OSC can vary according to Allium species [22], plant cultivation or storage conditions and processing methods [23]. Some OSC are absent in the bulbs and require mechanical exposure like cutting, crushing or chewing to be synthesized. According to Verma [24], whole garlic bulbs contain 16 OSC versus the 23 OSC of the crushed bulbs [24]. The cytoplasm of intact cloves contains biologically inactive γ-glutamylcysteine and S-alkylcysteine sulfoxides that serve as precursors for the volatile thiosulfinates [25,26]. Due to their high instability, the volatile thiosulfinates are degraded, within 24 hours, into both “second generation products” like oil-soluble mono-, di- and triallylsulfides (DAS, DADS, DATS) and vinyldithiins, thioacroleines, as well as ajoene [27-29]. These possess considerable biological activity and thus, possibly, represent the actual biologically active compounds [25]. Since garlic is more often consumed in a cooked, or dried, rather than raw state, the effects of these processing methods on its organosulfur compounds raise several questions and important concerns. As heat inactivates enzymes, it is generally accepted that heating garlic cloves inactivates alliinase and consequently prevents the formation of both allicin and other thiosufinates [29]. Several studies have been conducted to assess the active metabolites of garlic. Experimental data suggest that each activity is strongly dependent on the used methods [30] and the health benefits can also vary with preparation [31]. As a result, the different garlic formulations available at the market vary in their chemical composition.
In view of the differences in the organosulfur composition of different garlic preparation, the aim of the present study is to elucidate the analytical behaviour of OSC, by evaluating the impact of heating temperature and time on the OSC composition of A. sativum. Mainly, the formation of mono-, di- and trisulfides was studied in order to reveal the variation of OSC, conducted by the variation of analytical parameters using an experimental design.
Plant material
Plants of A. sativum cv. “Softneck” were randomly collected in October 2012 from a cultivated population, in Manouba-Tunisia.
Experimental design
The effect of heating temperature and heating time, as variables, on the production of OSC was evaluated in a 22 factorial design (Table 1).
| Experiment | Run | Ta | tb |
|---|---|---|---|
| 1 | 3 | 30 | 3 |
| 2 | 2 | 120 | 35 |
| 3 | 1 | 30 | 35 |
| 4 | 4 | 120 | 3 |
Table 1: Independent variables and design layout in the 22 factorial design.
The behavior of the system was described using the following second-order polynomial:

Where, Y is the predicted response, ao is the interception coefficient, a1 are the linear terms, a12 are the interaction terms, and T and tmin are the coded levels of the independent variables temperature and time, respectively. The models of the two responses were expressed in terms of coded variables, without taking into account the statistically insignificant terms.
Table 1 represents the variables of interest and their real values at the levels set in the design. Temperature levels were 35°C and 120°C; times were 3 and 30 min. In the design matrix, the effects of two variables on formation of selected OSC were evaluated. The runs were randomized for statistical purposes. Then, the data from run 1 to run 4 were computed and plotted using Design Minitab 17 software. The most important Headspace-GC-MS variables were selected based on the literature and preliminary tests undertaken to assess the tendencies of the factors and which had the greatest influence on responses for selected OSC.
Analytical procedure
Two grams of garlic slices, previously homogenized, were weighed into 15 ml screw-cap aroma headspace vials, which were closed with an aluminum seal. The vial was placed onto a magnetic stirrer. Headspace extraction was carried out immediately after sample preparation. Volatiles were extracted by exposing the solid-phase micro extraction (SPME) fiber to the headspace (HP 7694) of a sample vial. The compounds extracted were withdrawn through the septum by a gas syringe and analyzed by gas chromatography.
The GC-MS analysis was carried out on an Agilent 6890N Net Work GC System model equipped with a DB-5 column (JandW Scientific, USA) at dimensions of 50 m; 0.32 mm ID and 0.25 mm film thickness. Average helium carrier gas flow rate: 1 ml/minutes, column split ratio: 50:1, injector and detector temperatures: 200°C and 230°C, respectively. The column oven temperature was held at 60°C for 1.5 minutes, then programmed to 100°C at 2°C/min and then to 150°C at 10°C/min. The injected volume was 1 ml. The ionization energy was at 70 eV with a scan time of 1 s and a mass range of 40-300 amu. The components of the garlic preparation were identified by comparing their mass spectra with those of a computer library (Wiley 27 L). The relative percentages of components were obtained by peak-area normalization without correction factors.
Temperature and time incubation were evaluated and optimized by means of a factorial design and the obtained results were evaluated by an analysis of variance (ANOVA).
Analysis of the mode l
Table 2 shows the main volatile compounds of garlic obtained after different treatments. The most common allyl sulfides were diallyl disulfide (DADS), diallyl sulfide (DAS), diallyl trisulfides (DATS) and methyl allyl trisulfides (MATS) as found previously by Kalra et al. [13]. The pharmacological activity of garlic has been attributed to the presence of organosulfur compounds, mostly related to oil-soluble allyl sulfides such as DATS, DADS and DAS. Allyl sulfides showed valuable action in the liver, which could be used for protection of normal liver cells or for improvement of liver damage. Numerous researches have approved the antioxidant activity of DAS as it suppressed cytotoxicity and oxidative tissue damage by increasing antioxidant enzyme activities and decreasing lipid peroxidation. Therefore, it can be used as a dietary preventive agent [6]. The results of GC-MS analysis of the garlic constituents showed that heating increased the proportions of some volatile compounds in garlic, such as DAS (from 0 to 14.70%), methyl allyl trisulfide (from 0 to 14%) and diallyl trisulfide (from 0 to 18.30%). The methyl allyl disulfide and diallyl disulfide contents increased after the heat treatment at 120°C by 14% and 86%, respectively. The effect of heat treatment at 35°C was less explicit with an increase of 5% and 42.40%, respectively. It was observed that dimethyl sulfide (DMS), constituents completely vanished at 35°C for 3 min and at 120°C for 30 min. However, Matan et al. [32] showed that heat curing at 100°C increased the proportion of diallyl disulfide from 38.86 to 53.29, while it slightly reduced proportions of other constituents, such as diallyl sulfide (from 19.81 to 15.22) as compared to the effect of 30°C treatments. It can be concluded that heat (at high temperatures) enhanced the formation of diallyl sulfide and simultaneously slightly induced the decomposition of diallyl disulfide. Transformation also seems to lead to the formation of polysulfide’s that are present in multiple garlic preparations in substantial quantities and are comparatively stable [13]. DAS is an active organic sulphur compound derived from garlic that can suppress cytotoxicity induced by chemicals in animal models and is well-known for its antioxidant properties. DAS and related compounds have been shown to suppress oxidative tissue damage by increasing antioxidant enzymes activities and decreasing malondialdehyde level in lung and kidney tissues [6].
| Organo-Sulfur Compounds | Relative content (%) at | ||||
|---|---|---|---|---|---|
| (35°C, 3 min) | (35°C, 30 min) | (120°C, 3 min) | (120°C, 30 min) | ||
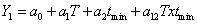 |
Methyl allyl sulfide (MAS) | 0 | 0.52 | 2.50 | 5.70 |
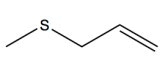 |
Dimethyl sulfide (DMS) | 0 | 0.80 | 0.80 | 0 |
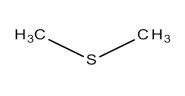 |
Diallyl sulfide (DAS) | 0 | 0 | 3.20 | 14.70 |
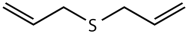 |
Methyl allyl disulfide (MADS) | 14 | 22.70 | 12.9 | 5 |
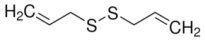 |
Diallyl disulfide (DADS) | 86 | 76 | 69.10 | 42.40 |
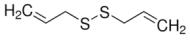 |
Methyl allyl trisulfide (MATS) | 0 | 0 | 5 | 14 |
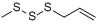 |
Diallyl trisulfide (DATS) | 0 | 0 | 6.60 | 18.30 |
| Total monosulfides | 0 | 0.52 | 5.7 | 20.4 | |
| Total disulfides | 100 | 99.5 | 82.8 | 47.4 | |
| Total trisulfides | 0 | 0 | 11.6 | 32.3 | |
Table 2: Effect of heating temperature and heating time on main OSC of garlic (A. sativum).
Sulfur compounds are also present in garlic oil. They can be classified into two main groups according to their cyclic (contains the two vinyldithiin isomers, e.g., 2-vinyl-1,3-dithiane) or acyclic (consists of various mono- to polysulfide’s, e.g., diallyl disulfide and diallyl sulfide) structures [33]. Detection of diallyl disulfide and diallyl trisulfides after heating at high temperature was in accordance with the finding of Kimbari [33] and Abu-Lafi et al. [34], who showed that formation of various acyclic compounds from garlic could be promoted by heat. A high level of acyclic compounds was observed in an extraction procedure at high temperatures, while extraction at room temperature greatly reduced and increased levels of acyclic and cyclic compounds, respectively [33]. Acyclic compounds, such as diallyl disulfide, are formed by thermal degradation of thiosulfinates previously produced by garlic’s unstable main precursor, allicine (Figure 1) [35]. According to these authors, diallyl disulfide can oxidize back to allicin and this might explain a reduction in the proportion of diallyl disulfide after 30 min of incubation at 120°C temperature.
In view of the fact that garlic is normally consumed in cooked foods and as dried spice, it has been also investigated by several authors that heating might affect the chemical composition of garlic including its antioxidant properties. It has been also established that heating of garlic cloves for 60 s using microwaves reduces its anticancer properties [36] and the heating of garlic extracts for 10 min at 100°C reduces the ability to inhibit platelet aggregation [37]. In contrast, the OH* scavenging properties of garlic were essentially preserved when garlic extracts were heated [38]. Shobana and Naidu [39] found that the lipid peroxidation inhibiting capacity of garlic was not affected by boiling (30 min at 100°C). Recently, it was also found that the ability of garlic extracts to inhibit Cu2+induced lipoprotein oxidation in human serums was not affected by the heating whole garlic cloves or extracts of garlic powder [38]. By contrast, Yin and Cheng [40] found that heating treatment (100°C for 15 min in an oven) of chopped garlic reduced the ability to inhibit lipid peroxidation. Furthermore, the hydrogen peroxide radical scavenging ability of garlic decreased in the heat treated extract of garlic powder [38]. These findings indicates that the effect of heating on biological activities of garlic depend on the allinase activity and the consequent formation of thiosulfinates [22].
Main and interaction effects: Half-normal probability plot and diagram of Pareto
Quantitatively, the estimated effects of temperature and time gave main effect or interaction effect via least squares estimation (Figure 2). Based on these estimates we could construct a list of the main effects and interactions, according to the order of the effect magnitude. All the effects that lie along the line were negligible, whereas larger ones are far from the line. It could be established, the main effects including the heating temperature (A) effect, influenced the monosulfides and the trisulfides formation within the levels and conditions tested. The fit of the models was also checked by the diagram of Pareto. It could be confirmed that the predicted response values are in good agreement with the experimental data and that temperature exhibit the more important effects on production of trisulfides mainly. Higher polysulfides are formed at higher temperatures [26].
In accordance with this study, the following ranking can be established: monosulfides A>B>AB, disulfides A>AB>B and trisulfides A>AB>B, where A is the incubation temperature and B the incubation time (Figures 2 and 3).
ANOVA analysis
In order to ensure a good model, test for significance of the regression model and test for significance on individual model coefficients must be performed. An ANOVA table is commonly used to summarize the tests that were performed and to evaluate the statistical significance of the model. Table 3 shows the correlation, F-value and P-value of the main mono-, di- and trisulfides compounds as a response. The statistical significance of the models (time and temperature needed for the production of more OSC) was determined by an F-value and P-value. Corresponding P-values suggest that, among the test variables used in this study, a (temperature) is significant since P-values were less than 0.05 for the monosulfides and trisulfides. The fit of the models was checked by R2, which was found to be 0.995 and 0.998 for the produced trisulfides and the monosulfides, respectively. In fact, these values which were close to 1 were acceptable and indicated that there was an adequate goodness-of-fit. A small value of R2 indicated a poor relevance of the dependent variables in the model [41]. The P-value is used as a tool to check the significance of each coefficient, which also indicated the interaction strength between each independent variable.
| Organo-Sulfur Compounds | R2 model | F-Value | P-value | |
|---|---|---|---|---|
| Monosulfides | 0.995 | T (°C) | 185.33 | 0.047 |
| t (min) | 1.32 | 0.456 | ||
| Disulfides | 0.623 | T (°C) | 1.14 | 0.48 |
| t (min) | 0.51 | 0.605 | ||
| Trisulfides | 0.998 | T (°C) | 467.5 | 0.029 |
| t (min) | 1 | 0.5 |
Table 3: ANOVA results for time period and temperature needed for the production of organo-sulfur compounds.
The evaluation of the obtained results by analysis of variance (ANOVA) (Table 3) showed that temperature has a direct statistically significant effect on the monosulfides and trisulfides formation (P<0.05). The time was non-significant for sulfides formation or decomposition (P>0.05). Heat appeared to have positively affected the production of organosulfur compound by garlic and this temperature dependence was more pronounced for monosulfides and trisulfides. In fact, increased time and increased temperature showed negative main effects on disulfides concentration but a significant (P<0.05) positive effect on mono- and trisulfides formation. According to Figure 3, heating temperature (120°C) appeared to strongly enhance the production of monosulfides and increasing from 0 to 20.4% and from 0 to 32.3% for trisulfides. In contract to the situation with monosulfides and trisulfides, heating temperature affect the decomposition of disulfides. These result was confirmed by the corresponding correlation values given on Table 4, suggesting that composition of monosulfides and trisulfides was much correlated (P<0.05 and R2=0.98).
| Correlation between | R2 | P-value |
|---|---|---|
| Mono- and disulfides | 0.569 | 0.431 |
| Di- and trisulfides | 0.702 | 0.298 |
| Mono- and trisulfides | 0.984 | 0.016 |
Table 4: Correlation between organo-sulfur compounds.
From a combination of the process variables estimated and the ANOVA results, a polynomial model with statistical significance can be generated. This model, quantitatively elucidating the effects of process variables with statistical significance, is presented as follows: In order to maximize mono, di and trisulfides, which were considered as target responses, values of linear model 22 calculated by ANOVA are given below:
Monosulfides=-146616+4175 T - 715.5 tmin+24.96 T × tmin
Disulfide=-2316248+67099 T+102315 tmin - 2172 T × tmin
Trisulfides=-306309+8752 T+993.2 tmin - 28.38 T × tmin
Given the complexity of the chemical composition of garlic, our proposed new analytical procedure can be a useful alternative to predict the presence of OSC in garlic, with regards to the preparation conditions (specifically temperature and time) and method. Effects of temperature and time as operational parameters were investigated on the organosulfur production of garlic. In the present work, we demonstrate that the OSC composition of A. sativum is due not only to edaphoclimatic conditions but also to the chromatographic parameters. The 22 factorial design experiment used in this study was found to be very effective in the evaluation of OSC formation or decomposition, as a function of time and temperature. Increased temperature indicated negative main effects on disulfides concentration, and by a contrast, a positive effect on mono and trisulfides formation. We can deduce that disulfides are thermally labile compounds and mono- and polysulfides are their breakdown products.