Research Article - (2020) Volume 8, Issue 1
Curcumin is a substance in turmeric. It’s an active compound from natural plant Curcuma longa L which is widely used as an herbal medicine in Asian countries. The anti-inflammatory, antioxidant and anticancer properties that slow down the spread of cancer, makes chemotherapy more effective and protect healthy cells from damage by radiation therapy. However, it’s poor water, plasma solubility and bioavailability for human absorption. To overcome these problems, structural modification is attracting scientist’s attention.
In the present study, several curcumin derivatives including ferulic acid curcumin, isovanillin curcumin, 4-methoxy- 1-naphthaldehyde curcumin, 3,5-di-tert-butyl-4-hydroxybenzaldehyde curcumin, syringaldehyde curcumin, ibuprofen curcumin were designed and synthesized successfully with the percentage yield of 15.0%, 29.6%, 52.0%, 23.0%, 13.0% and 11.0% respectively.
Turmeric; Curcuma longa L; Structural modification; Curcumin derivatives
Curcumin is an active compound from the traditional herbal medicine Turmeric, Curcuma longa L (Figure 1) [1]. The chemical structure of curcumin is 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6- heptadiene-3,5-dione (Figure 2) with chemical formula C21H20O6 and molecular weight of 368.38 gmol-1 [2]. It consists of different tautomerism including Bis-keto and enolate forms. The Bis-keto form is more predominant than the enolate form under acidic and neutral conditions [3].

Figure 1. Curcuma longa L.
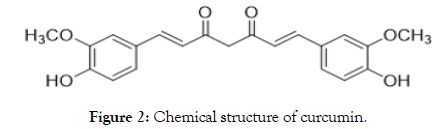
Figure 2. Chemical structure of curcumin.
Curcumin is non-toxic and has a variety of positive pharmacological effect such as anti-oxidative, anti-inflammatory, antiseptic and anticancer properties (Figures 3 and 4) [4]. It inhibits the tumor formation, promotion, progression and dissemination in the animal model. Nowadays, curcumin used as an alternative medicine agent for the treatment of some common diseases including arthritis and skin infection [5]. It has been recognized by Food and Drug Administration (FDA) in USA and World Health Organization [6].
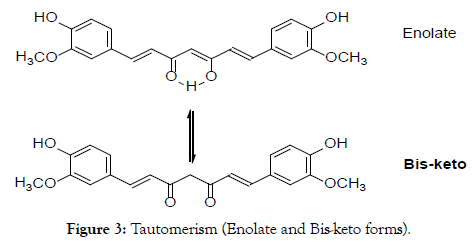
Figure 3. Tautomerism (Enolate and Bis-keto forms).
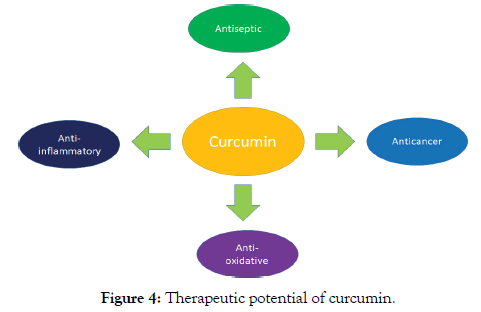
Figure 4. Therapeutic potential of curcumin.
According to the previous studied, even doses as high as 8 g of curcumin per day administered to human subjects. The average serum concentration only has 652.5 ng/ml [7]. It’s is a very lower absorption in the human body because of the poor water and plasma solubility.
In fact, curcumin consists of two phenolic groups and one 1,3-dikentone group which can be used for chemical modification (Figure 5) to improve the water, plasma solubility and maintain their biological activities. Actually, this is not all position suitable for modification. Phenol group controls the water and plasma solubility; Bis-keto group is associated with the anti-oxidant properties in curcumin, therefore, we must keep the enol group, just modify the phenol and bis-keto groups only (Figure 6) [8].
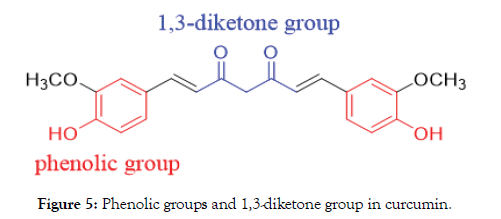
Figure 5. Phenolic groups and 1,3-diketone group in curcumin.
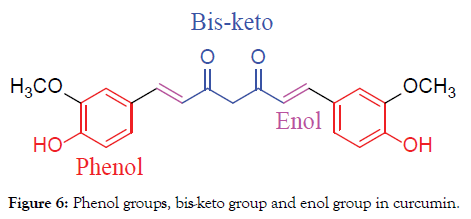
Figure 6. Phenol groups, bis-keto group and enol group in curcumin.
In the present study, curcumin is modified by introducing ferulic acid, isovanillin, 4-methoxy-1-naphthaldehyde, 3,5-di-tert-butyl-4- hydroxybenzaldehyde, syringaldehyde and ibuprofen. The above modifications are mainly based on the following concerns:
Ferulic acid is an antioxidant containing reactive free radicals such as reactive oxygen species (ROS). It implicates in DNA damage cancer and accelerated cell aging. It has the antitumor activity against breast cancer and liver cancer because of the pro-apoptotic effects in cancer cells leading to their destruction [9].
Isovanillin is the phenolic aldehyde and isomer of vanillin. It is metabolized by aldehyde dehydrogenase into isovanillic acid, which acts as selective inhibitor of aldehyde oxidase and inhibits prostate cancer and reduces the growth rate of prostate tumors [10].
4-methoxy-1-naphthaldehyde is fluorogenic substrate in human alcohol dehydrogenase (ADH). It is produced in human pancreas and liver homogenates. The alcohol dehydrogenase (ADH) also lowers the opportunity of breast cancer in normal mammary tissues [11].
3,5-di-tert-butyl-4-hydroxybenzaldehyde acts as potent analgesic and possesses anti-inflammatory activities in cancer cells [12]. Syringaldehyde moderately inhibits COX-2 activity and also has anti-hyperglycemic and anti-inflammatory activities [13].
Ibuprofen is used for treating pain, fever and inflammation. It inhibits the production of prostaglandins by decreasing the activity of the enzyme cyclooxygenase in order to lower the chance of breast cancer [14]. Thus, several curcumin derivatives including ferulic acid curcumin, isovanillin curcumin, 4-methoxy-1-naphthaldehyde curcumin, 3,5-di-tert-butyl-4-hydroxybenzaldehyde curcumin, syringaldehyde curcumin, ibuprofen curcumin were designed and synthesized (Figure 7).
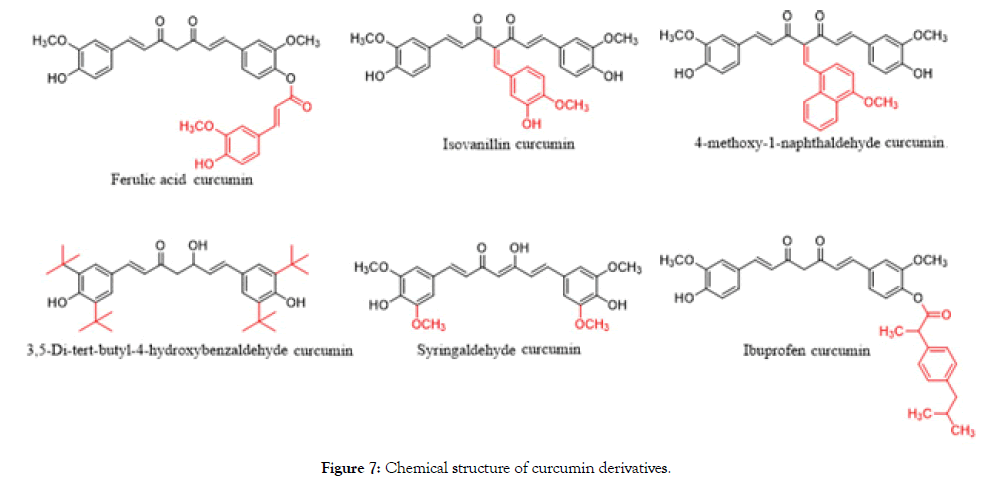
Figure 7. Chemical structure of curcumin derivatives.
Materials
Curcumin, Trans-Ferulic acid, 3-hydroxy-4-methoxybenzaldehyde, 4-methoxy-1-naphthaldehyde, 3,5-di-tert-butyl-4- hydroxybenzaldehyde, Syringaldehyde and Ibuprofen were purchased from Sigma Aldrich in China Hong Kong. N,N'- Dicyclohexylcarbodiimide (DCC), 4-Dimethylaminopyridine (DMAP), Piperidine, Boric oxide, 2,4-Pentanedione, tributyl borate, EDCI, HOBT and DIEA were obtained from J&K scientific company in Beijing, China. All the other agents were analytical grade and were used without further purification. Deionized (DI) and ultra-pure water (UPW) were purified by a Milli-Q system (Millipore, USA).
Generally, there were four major steps including (1) dissolved the starting materials; (2) added the catalyst and coupling reagent; (3) extraction as well as the (4) purification.
Synthesis of ferulic acid curcumin
Curcumin (19.16 g, 0.052 mol) and Trans-Ferulic acid (10.10 g, 0.052 mol) were dissolved in 1,2-dichloroethane (200 mL) and stirred. N,N'-Dicyclohexylcarbodiimide (DCC) (16.09 g, 0.078 mol) and 4-Dimethylaminopyridine (DMAP) (6.35 g, 0.052 mol) were added. After stirring at 85°C for 24 h, the reaction mixture was filtered and the filtrate was washed with distilled water (150 mL). It was extracted with dichloromethane twice times (2 × 50 mL) and the combined organic layers were washed with brine (100 mL) and dried over sodium sulfate. Solvent (dichloromethane) was evaporated under reduced pressure. The crude residue was purified by chromatography with silica gel through dichloromethane (DCM) to afford the pure orange color compound (4.2 g, 15.0%) (Figure 8).
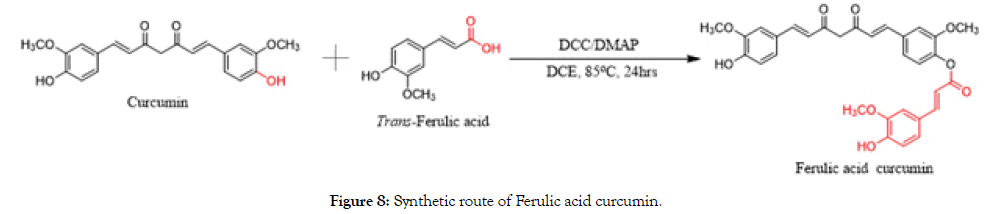
Figure 8. Synthetic route of Ferulic acid curcumin.
Synthesis of isovanillin curcumin
Curcumin (15.0 g, 40.7 mmol) and 3-hydroxy-4-methoxybenzaldehyde (6.20 g, 40.7 mmol) were dissolved in the toluene (300 mL) and DMF (50 mL) equipped with a water dispenser. Piperidine (693 mg, 8.14 mmol) and acetic acid (488 mg, 8.14 mmol) were added as a catalyst. The reaction mixture was stirred at 140°C for overnight and the generated water was removed by water dispenser. It’s was poured into water, extracted with ethyl acetate and washed with brine. Filtrate was dried over sodium sulfate and concentrated. The residue was purified by silica gel column chromatography through 1:2 petroleum ether and ethyl acetate followed by recrystallization in the same ratio mixture of toluene and ethyl acetate to afford the pure yellow solid (6.00 g, 29.6%) (Figure 9).
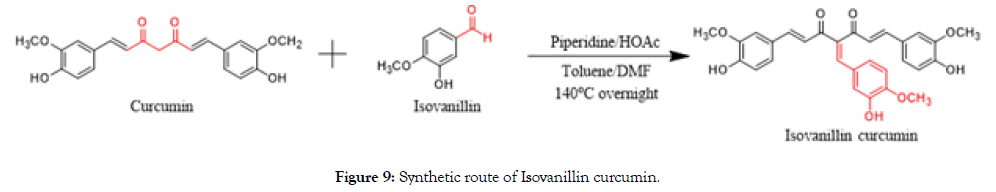
Figure 9. Synthetic route of Isovanillin curcumin.
Synthesis of 4-methoxy-1-naphthaldehyde curcumin
Curcumin (10.0 g, 27.0 mmol) and 4-methoxy-1-naphthaldehyde (5.00 g, 27.0 mmol) were dissolved in the toluene (1.4 L) equipped with a Dean-Stark apparatus. Piperidine (1.00 g, 11.8 mmol) and acetic acid (0.70 g, 11.8 mmol) were added as a catalyst. The reaction mixture was stirred at 150°C for overnight. It’s was poured into water, extracted with ethyl acetate and washed with brine. Filtrate was dried over sodium sulfate and concentrated. The residue was purified by silica gel column chromatography through 1:2 petroleum ether and ethyl acetate to afford the pure yellow solid (7.50 g, 52.0%) (Figure 10).
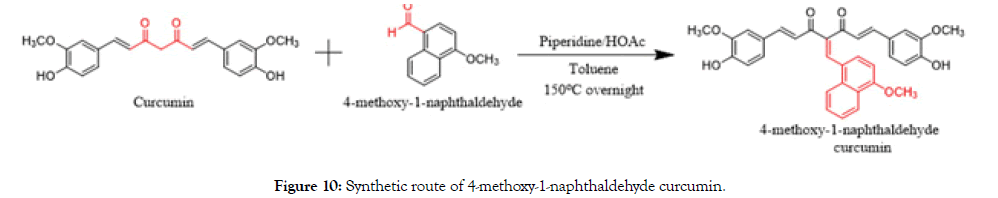
Figure 10. Synthetic route of 4-methoxy-1-naphthaldehyde curcumin.
Synthesis of 3,5-di-tert-butyl-4-hydroxybenzaldehyde curcumin
Boric oxide (7.0 g, 0.1 mmol, 0.5 eq) was dissolved in DMF (250 mL) and added to 2,4-Pentanedione (10.2 mL, 0.1 mmol, 0.5 eq), followed by tributyl borate (54.0 mL, 0.2 mmol, 1.0 eq) at 65°C and stirred for 15 min. To the above borate complex, 3,5-Di-tert-butyl- 4-hydroxybenzaldehyde (46.8 g, 0.2 mmol, 1.0 eq) was added and stirred for 5 min. A mixture of 1,2,3,4-tetrahydroquinoline (3 mL) and acetic acid (8 mL) in DMF (25 mL) was added to the reaction mixture and heated to 95°C for 4 h. After cooling to 15°C, acetic acid (20%, 150 mL) was added with stirring and again the reaction mixture was stirred at 70°C for another 1 h. It was cooled to 15°C and the so-formed solid was filtered, washed with water (250 mL) and dried. The crude was purified by flash column chromatography on silica gel eluting with petroleum ether/dichloromethane (DCM) from the ratio of 50:1 to 10:1. 45 g of wine red solid was produced. It was triturated with petroleum ether/methanol (2 × 50 mL) to get the desired compound as an orange solid (25 g, 23.0%) (Figure 11).
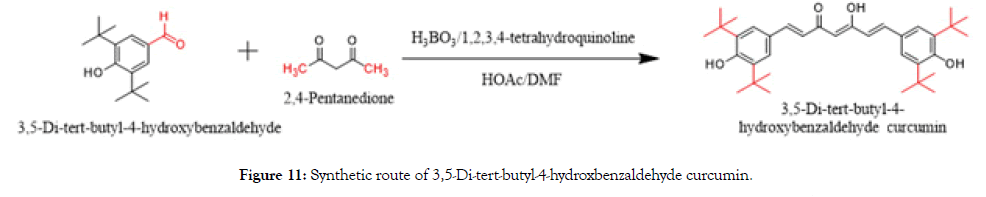
Figure 11. Synthetic route of 3,5-Di-tert-butyl-4-hydroxbenzaldehyde curcumin.
Synthesis of syringaldehyde curcumin
Boric oxide (11.5 g, 165 mmol, 0.5 eq) was dissolved in DMF (400 mL) and added to 2,4-Pentanedione (16.8 mL, 165 mmol, 0.5 eq), followed by tributyl borate (89.1 mL, 330 mmol, 1.0 eq) at 65°C and stirred for 15 min. To the above borate complex, syringaldehyde (60 g, 330 mmol, 1.0 eq) was added and stirred for 5 min. A mixture of 1, 2, 3, 4-tetrahydroquinoline (4 mL) and acetic acid (12 mL) in DMF (40 mL) was added to the reaction mixture and heated to 95°C for 4 h. After cooling to 15°C, acetic acid (20%, 200 mL) was added with stirring and again the reaction mixture was stirred at 70°C for another 1 hr. It was cooled to 15°C and the so-formed solid was filtered, washed with water (250 mL) and dried. The crude was purified by flash column chromatography on silica gel eluting with petroleum ether/dichloromethane (DCM) from the ratio of 50:1 to 10:1). 35 g of wine red solid was produced. It was triturated with petroleum ether/ methanol (2 × 50 mL) to get the desired compound as an orange solid (18 g, 13.0%) (Figure 12).
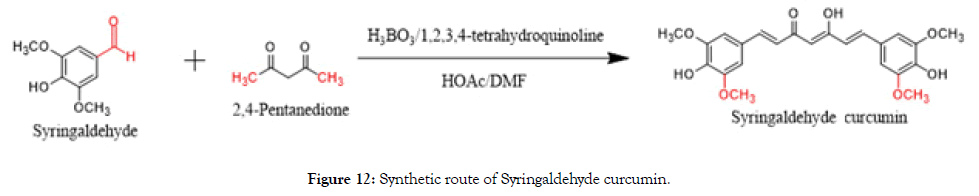
Figure 12. Synthetic route of Syringaldehyde curcumin.
Synthesis of ibuprofen curcumin: Ibuprofen (20 g, 0.1 mol) was dissolved in chloroform (100 mL) and added to EDCI (18.6 g, 0.12 mol), HOBT (16.2 g, 0.12 mol) and DIEA (25.8 g, 0.2 mol). After stirring at 0°C for 1 h, a solution of curcumin (36.8 g, 0.1 mol) in chloroform (150 mL) was added dropwise to the reaction mixture which was stirred overnight at room temperature. After completion of the reaction, the mixture was washed with hydrochloric acid (1 M) and brine. The organic layer was dried over anhydrous sodium sulfate and concentrated in vacuum. It’s was purified by column chromatography over polyamide gel in the ratio of 1:10, methanol/tetrachloromethane and triturated with petroleum ether/methanol (2 × 50 mL) to get the desired compound as a yellow solid (6.2 g, 11.0%) (Figure 13).
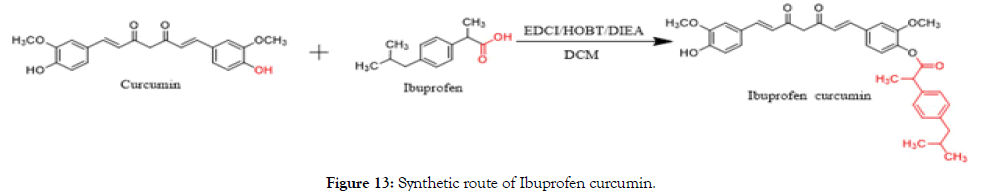
Figure 13. Synthetic route of Ibuprofen curcumin.
These curcumin derivatives including ferulic acid curcumin, isovanillin curcumin, 4-methoxy-1-naphthaldehyde curcumin, 3, 5-di-tert-butyl-4-hydroxybenzaldehyde curcumin, syringaldehyde curcumin and ibuprofen curcumin were characterized using the 1H NMR, 13C NMR, High resolution EMI-MS, High-performance liquid chromatography (HPLC) or Elemental analysis and UV-Vis spectrum analysis.
Ferulic acid curcumin
1H NMR (400 MHz, CDCl3): δ=3.89 (s, 3 H, OCH3), 3.95 (s, 6 H, OCH3), 5.90 (d, J=Hz, 2 H, CH2), 6.50 (d, J=Hz, 2 H, CHCHCO), 6.55 (d, J=Hz, 1 H, CHCHCOO), 6.95 (t, J=Hz, 2 H, ArH), 7.06 (s, 1 H, ArH), 7.10 (s, 1 H, ArH), 7.12-7.21 (m, 5 H, ArH), 7.61 (d, J=Hz, 1 H, CHHCO), 7.63 (d, J=Hz, 1 H, CHHCO), 7.82 (d, J=Hz, 1 H, CHCOO) (Figure 14).
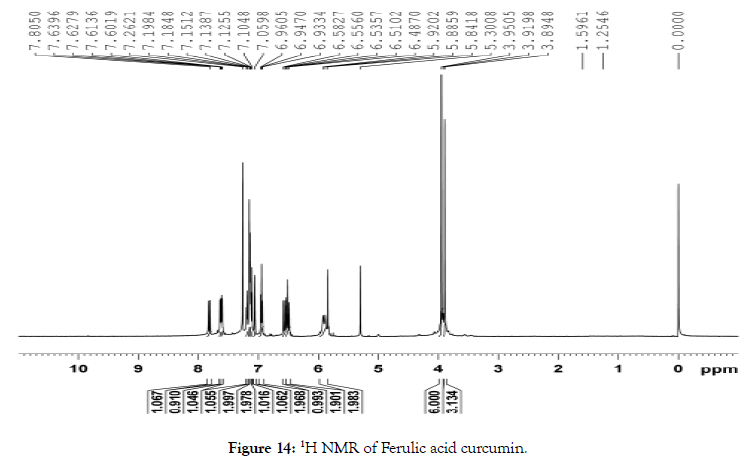
Figure 14. 1H NMR of Ferulic acid curcumin.
13C NMR (400 MHz, CDCl3): δ=184.59, 182.02, 165.22, 152.02, 151.69, 148.56, 148.13, 147.25, 146.97, 141.48, 141.24, 139.62, 134.09, 127.66, 126.85, 124.28, 124.07, 123.71, 123.57, 123.16, 121.87, 121.15, 114.95, 114.00, 111.58, 109.7, 101.69, 56.08, 50.42, 33.28, 29.82 (Figure 15).
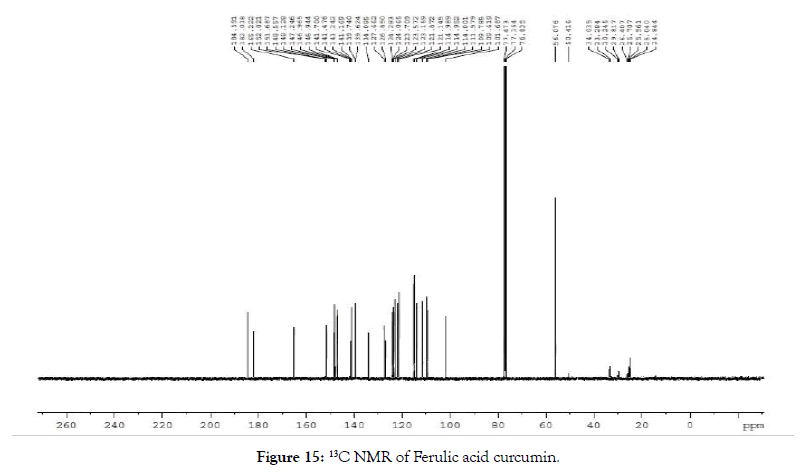
Figure 15. 13C NMR of Ferulic acid curcumin.
Elemental analysis
C (68.94%); H (5.275%) (Figure 16).
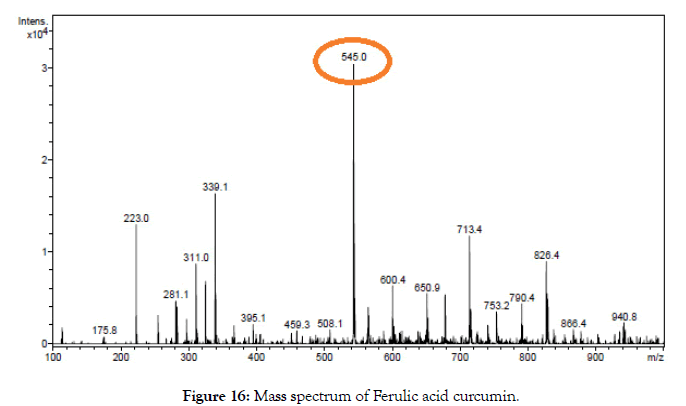
Figure 16. Mass spectrum of Ferulic acid curcumin.
ESI-MS (m/z) calculated for C31H28O9, 544.17; Found 545.00 [M+1] (Table 1).
| Weight [mg] | Method | C area | H area | C [%] | H [%] | C/H ratio |
|---|---|---|---|---|---|---|
| 2.038 | 2 mg | 29 158 | 7 112 | 68.94 | 5.28 | 13.0697 |
Table 1: Elemental analysis of Ferulic acid curcumin.
Isovanillin curcumin
1H NMR (400 MHz, CDCl3): δ=3.88 (d, 6H, OCH3), 3.92 (s, 3H, OCH3), 5.60 (d, J=Hz, s, 1 H, COCCH), 5.92 (s, J=Hz, 2H, CHCHCO), 6.78 (m, J=Hz, 2H, CHCHCO), 6.84 (d, 1 H, ArH), 6.89 (d, 1 H, ArH), 6.91 (d, 1 H, ArH), 6.97 (d, 2 H, ArH), 7.03 (t, 2 H, ArH), 7.08 (s, 1 H, ArH) 7.15 (d, 1 H, ArH), 7.45 (d, 1 H, Ar-OH), 7.74 (d, 1 H, Ar-OH), 7.77 (s, 1 H, Ar-OH) (Figure 17).
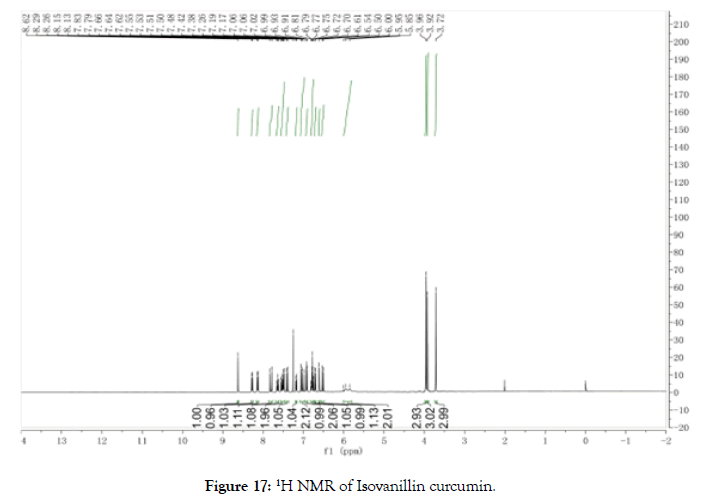
Figure 17. 1H NMR of Isovanillin curcumin.
13C NMR (400 MHz, CDCl3): δ=198.75, 186.97, 148.94, 148.7 2, 148.62, 147.62, 146.95, 146.92, 145.79, 145.38, 140.77, 139.12, 127.60, 127.15, 126.97, 125.76, 124.30, 123.90, 120.08, 116.31, 115.00, 110.76, 110.47, 110.15, 60.62, 56.30, 56.20, 56.16 (Figure 18).
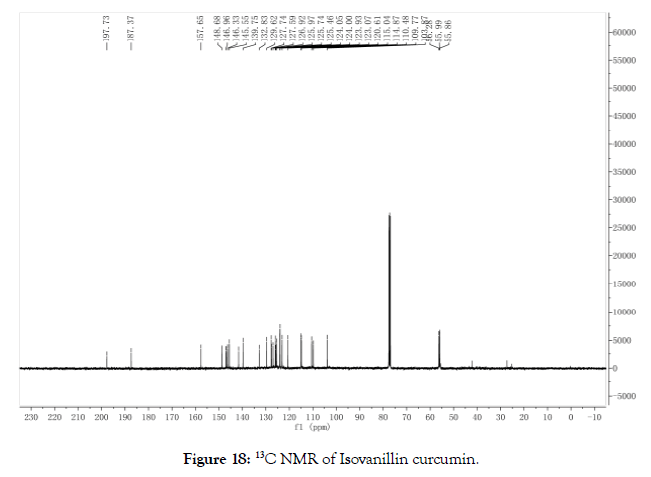
Figure 18. 13C NMR of Isovanillin curcumin.
HPLC: 220 nm and 250 nm, 0.04% TFA/H2O: 0.04% TFA/ ACN, >98% (Figures 19 and 20).
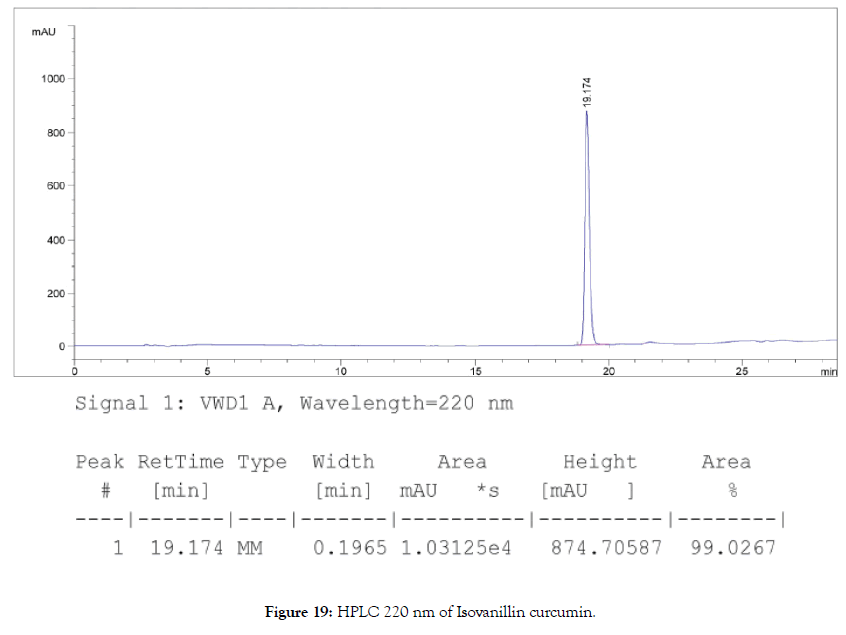
Figure 19. HPLC 220 nm of Isovanillin curcumin.
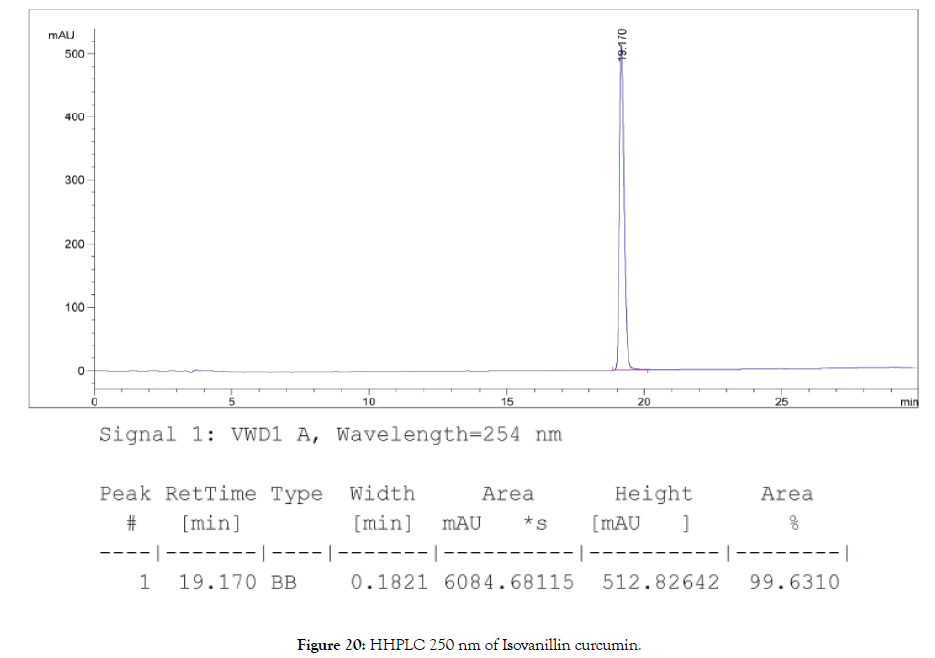
Figure 20. HHPLC 250 nm of Isovanillin curcumin.
ESI-MS (m/z): Calculated for C29H26O8: 502.16; Found: 503.20 [M+1] (Figure 21).
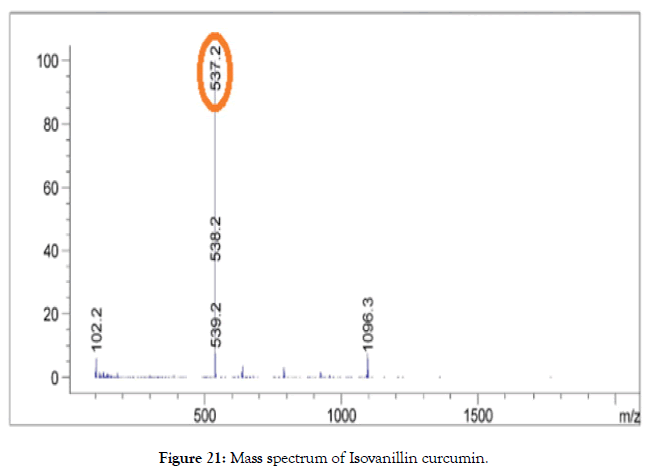
Figure 21. Mass spectrum of Isovanillin curcumin.
4-methoxy-1-naphthaldehyde curcumin
1H NMR (400 MHz, CDCl3): δ=3.72 (s, 3 H, OCH3), 3.92 (d, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.85-6.00 (m, J=Hz, s, 2 H, CHCHCO), 6.52 (d, J=Hz, 1 H, CHCHCO), 6.61 (s, J=Hz, 1 H, CHCHCO), 6.71 (d, 1 H, ArH), 6.78 (q, 2 H, ArH), 6.92 (d, 1 H, ArH), 7.03 (d, 2 H, ArH), 7.18 (d, 1 H, ArH), 7.40 (d, 1 H, ArH) 7.52 (m, 2 H, ArH), 7.64 (t, 1 H, ArH), 7.81 (d, 1 H, ArH), 8.14 (d, 1 H, Ar-OH), 8.28 (d, 1 H, Ar-OH), 8.62 (s, 1 H, COCCH) (Figure 22).
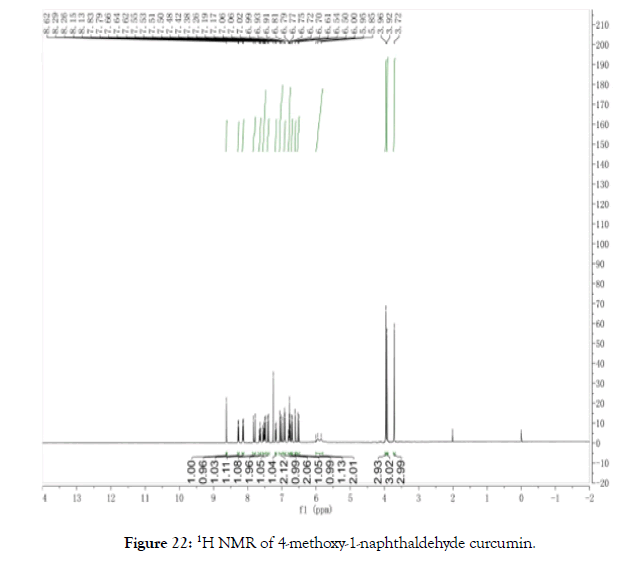
Figure 22. 1H NMR of 4-methoxy-1-naphthaldehyde curcumin.
13C NMR (400 MHz, CDCl3): δ=197.73, 187.37, 157.65, 148.68, 148.63, 146.96, 146.81, 146.33, 145.55, 141.58, 139.75, 132.83, 129.62, 127.74, 127.59, 126.92, 125.97, 125.74, 125.46, 124.05, 124.00, 123.93, 123.07, 120.61, 115.04, 114.87, 110.48, 109.77, 103.87, 56.28, 55.99, 55.87 (Figure 23).
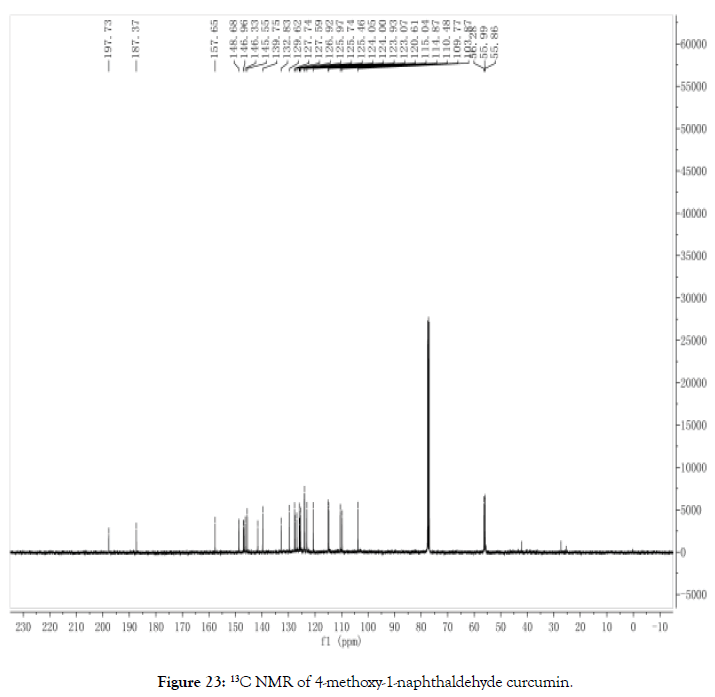
Figure 23. 13C NMR of 4-methoxy-1-naphthaldehyde curcumin.
HPLC: 220 nm and 254 nm, 0.04% TFA/H2O: 0.04% TFA/ ACN, >99% (Figures 24 and 25).
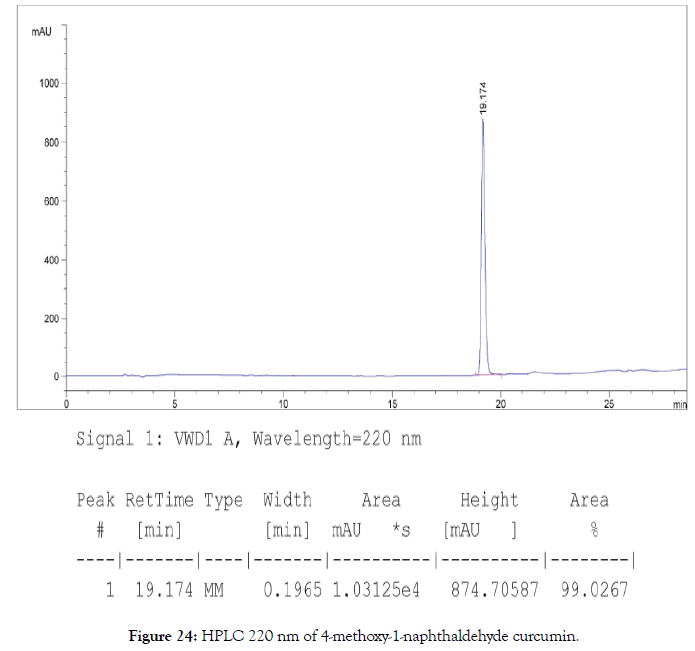
Figure 24. natural-products-chemistry-HPLC-8-367-g024
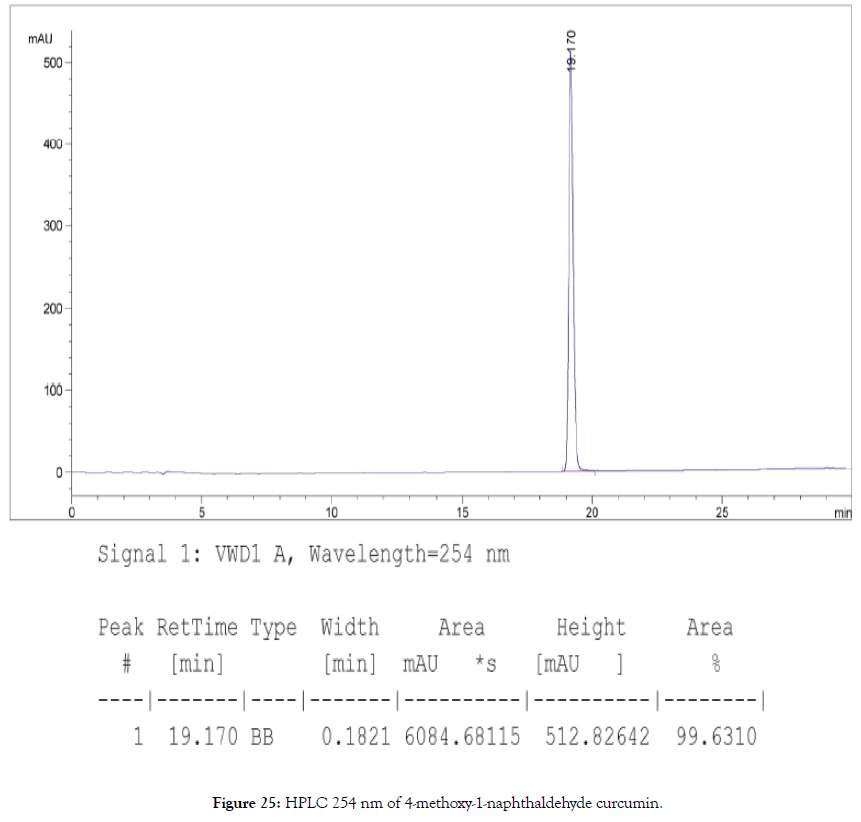
Figure 25. HPLC 254 nm of 4-methoxy-1-naphthaldehyde curcumin.
ESI-MS (m/z): calculated for C33H28O7: 536.18; Found: 537.20 [M+1] (Figure 26).
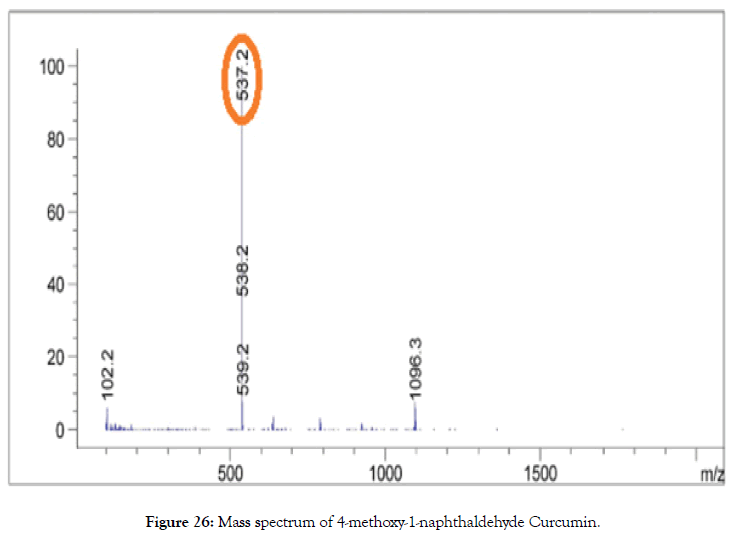
Figure 26. Mass spectrum of 4-methoxy-1-naphthaldehyde Curcumin.
3,5-Di-tert-butyl-4-hydroxybenzaldehyde curcumin
1H NMR (400 MHz, DMSO-d6): δ=1.35-1.41 (s, 36 H, CCH3), 6.24 (s, 1 H, CH=COH), 6.64-6.70 (d, 2 H, HOCCH=CH), 7.43 (s, 4 H, Ar-H), 7.49 (d, 2 H, HC=CHCO), 7.56-7.61 (s, 2 H, OCCH2=COH) (Figure 27).
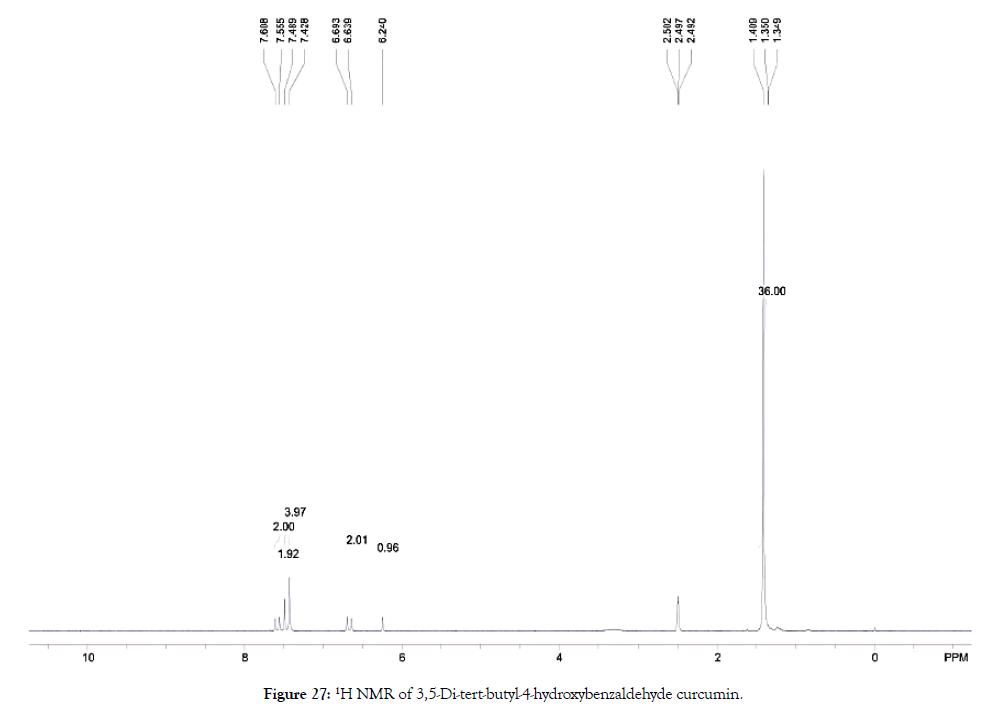
Figure 27. 1H NMR of 3,5-Di-tert-butyl-4-hydroxybenzaldehyde curcumin.
13C NMR (400 MHz, DMSO-d6): δ=30.58, 34.97, 100.96, 121.52, 125.67, 126.48, 139.61, 141.72, 156.93, 183.58 (Figure 28).
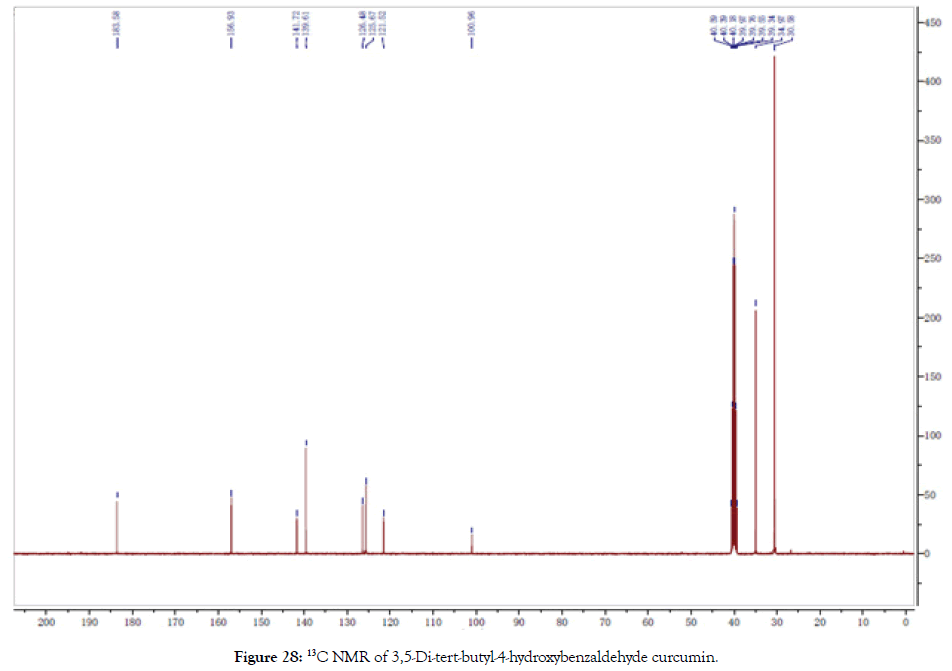
Figure 28. 13C NMR of 3,5-Di-tert-butyl-4-hydroxybenzaldehyde curcumin.
HPLC: 220 nm and 254 nm, 0.04% TFA/H2O: 0.04% TFA/ACN, >98% (Figures 29 and 30).
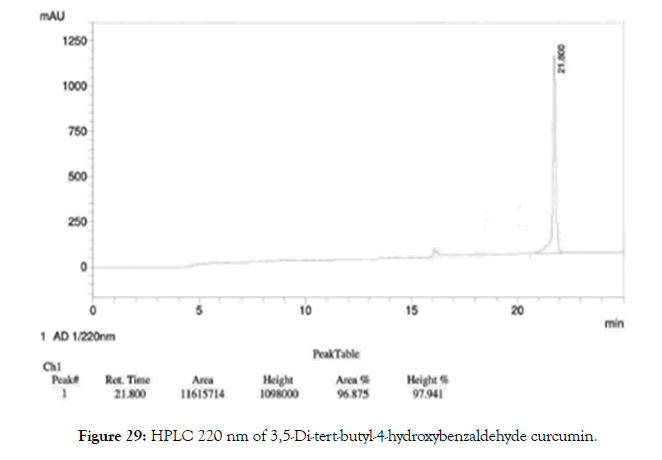
Figure 29. HPLC 220 nm of 3,5-Di-tert-butyl-4-hydroxybenzaldehyde curcumin.
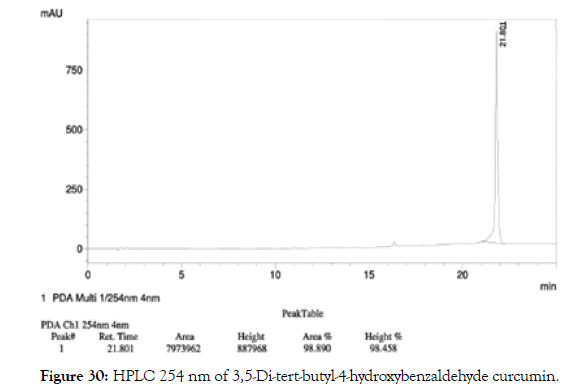
Figure 30. HPLC 254 nm of 3,5-Di-tert-butyl-4-hydroxybenzaldehyde curcumin.
ESI-MS (m/z): calculated for C35H48O4: 532.36; Found: 533.00 [M+1] (Figure 31).
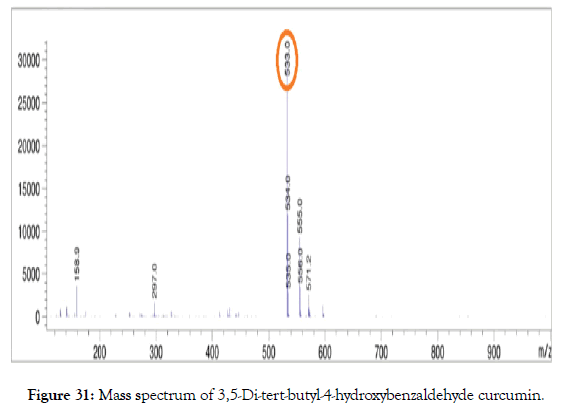
Figure 31. Mass spectrum of 3,5-Di-tert-butyl-4-hydroxybenzaldehyde curcumin.
Syringaldehyde curcumin: 1H NMR (400 MHz, DMSO-d6): δ=3.81-3.83 (s,12 H, Ar-OCH3), 6.09 (s, 1 H, CH=COH), 6.79-6.83 (d, 2 H, HOCCH=CH), 7.05(s, 4 H, Ar-H), 7.55-7.58 (d, 2H, HC=CHCO), 9.02 (s, 2 H, OCCH2=COH) (Figure 32).
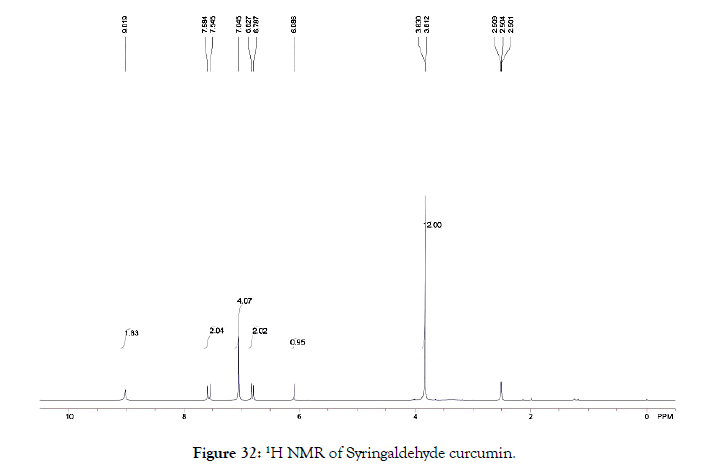
Figure 32. 1H NMR of Syringaldehyde curcumin.
13C NMR (400 MHz, DMSO-d6): δ=56.07, 100.75, 106.27, 121.48, 125.12, 138.40, 141.02, 148.10, 183.14 (Figure 33).
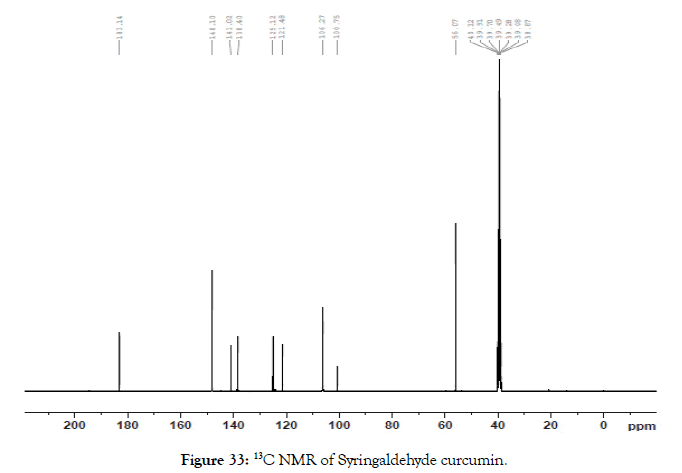
Figure 33. 13C NMR of Syringaldehyde curcumin.
HPLC: 214 nm and 254 nm, 0.04% TFA/H2O: 0.04% TFA/ACN, >99% (Figures 34 and 35).
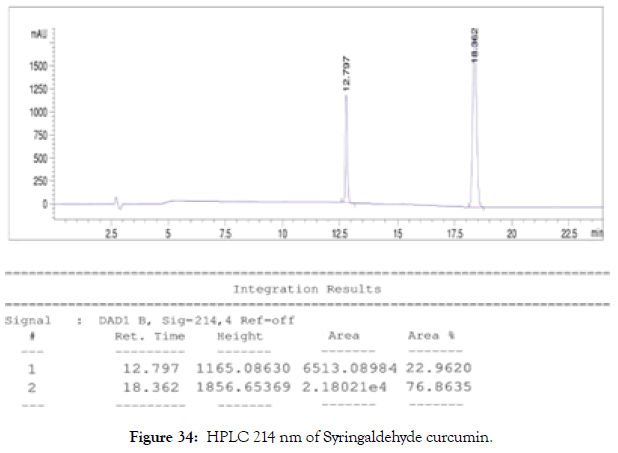
Figure 34. HPLC 214 nm of Syringaldehyde curcumin.
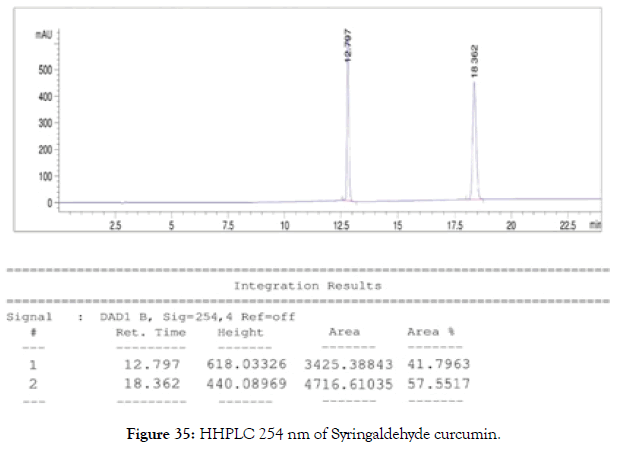
Figure 35. HHPLC 254 nm of Syringaldehyde curcumin.
ESI-MS (m/z): calculated for C23H24O8: 428.44; Found: 429.10 [M+1] (Figure 36).
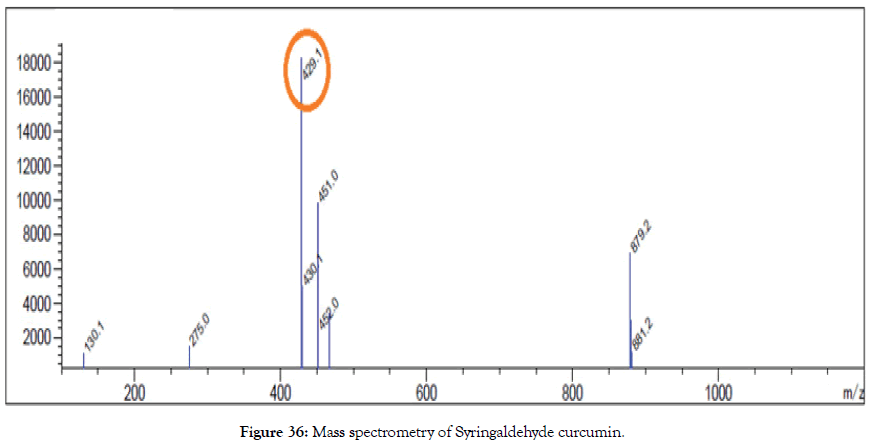
Figure 36. Mass spectrometry of Syringaldehyde curcumin.
Ibuprofen curcumin
1HNMR (400 MHz, DMSO-d6): δ=0.86-0.88 (d, 6 H, Ar-OCH3), 1.49-1.50 (d, 3 H, Ar-CH2CH(CH3)CH3), 1.80-1.87 (m, 1 H, OC=OCHCH2-Ar), 2.44-2.51 (t, 2 H, Ar-CH2CHCH2CH3), 3.75 (s, 3 H, H3CCH(CH3)CH2-Ar), 3.82-3.84 (s, 3 H, Ar-OC=OCH(CH3)-Ar). 4.03-4.08 (q, 1 H, Ar-CH2CH(CH3)CH3), 6.13 (s, 1 H, Ar-OC=OCH(CH3)-Ar), 6.78-6.84 (m, 2 H, OCCH=CH-Ar), 6.93-6.97 (d, 1 H, OCCH=CH-Ar), 7.05-7.07 (d, 1 H, OCCH=CH-Ar), 7.16-7.58 (m, 8 H, Ar-H), 7.61-7.62 (d, 2 H, HCOCCH2COCH), 9.73 (s, 1 H, Ar-OH) (Figure 37).
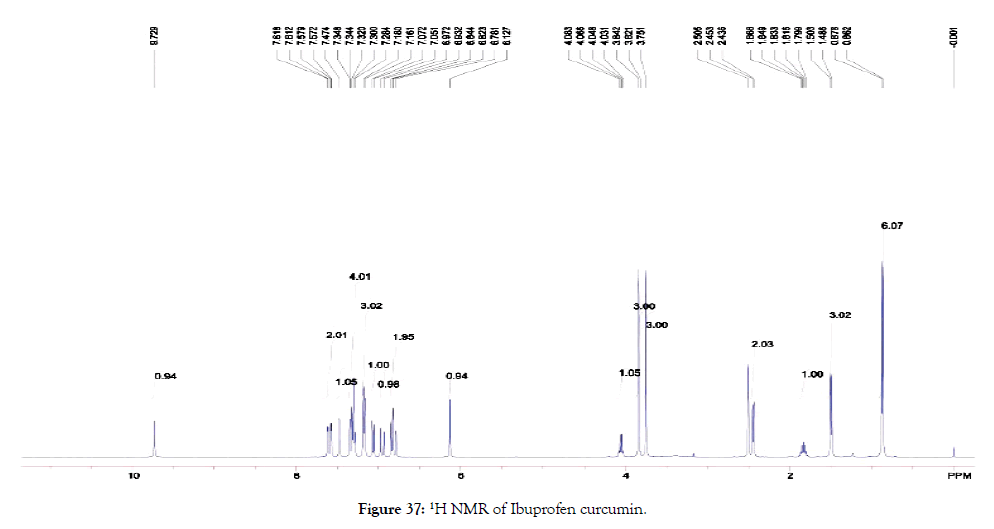
Figure 37. 1H NMR of Ibuprofen curcumin.
13CNMR (400 MHz, DMSO-d6): δ=19.22, 22.63, 22.66, 30.10, 39.28, 39.59, 39.79, 40.00, 40.21, 40.42, 56.17, 56.34, 101.79, 112.52, 116.18, 121.60, 121.72, 123.46, 123.82, 125.05, 126.68, 127.80, 129.55, 134.31, 137.80, 139.42, 140.43, 141.43, 142.03, 148.47, 150.03, 151.64, 172.57, 181.85, 185.36 (Figure 38).
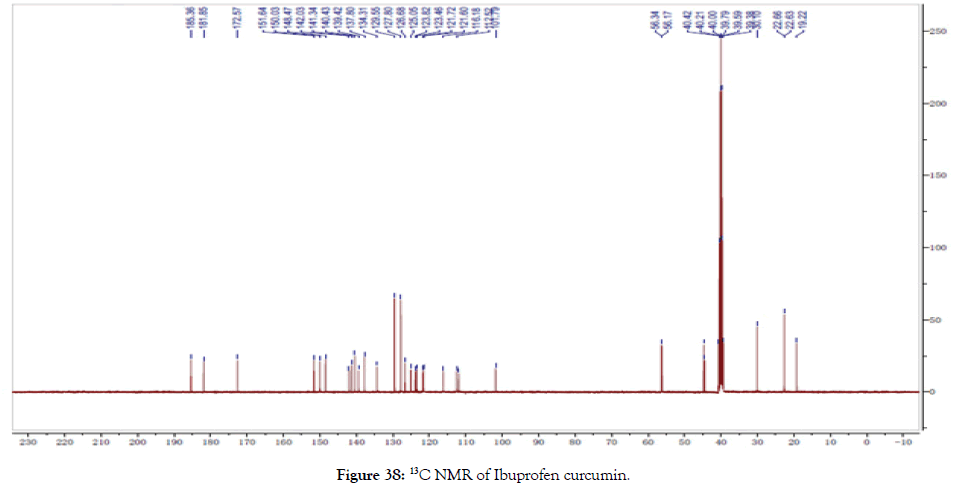
Figure 38. 13C NMR of Ibuprofen curcumin.
HPLC: 214 nm and 254 nm, 0.04% TFA/H2O: 0.04% TFA/ACN, >98% (Figures 39 and 40).
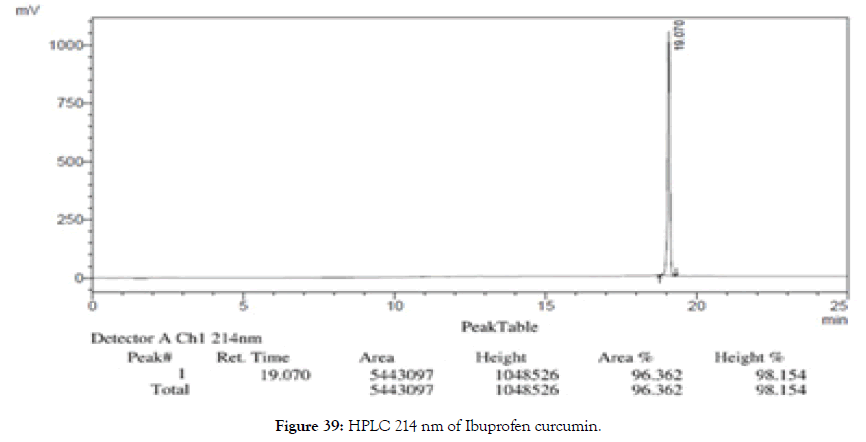
Figure 39. HPLC 214 nm of Ibuprofen curcumin.
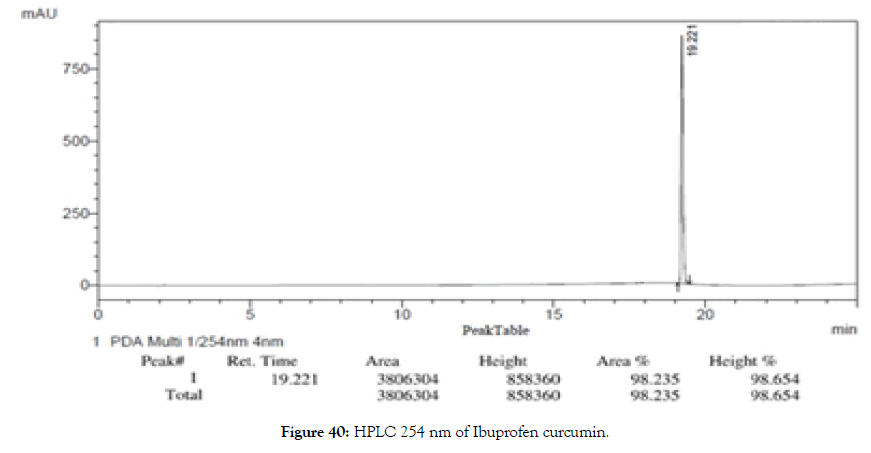
Figure 40. HPLC 254 nm of Ibuprofen curcumin.
ESI-MS (m/z): calculated for C34H36O7: 556.25; Found: 556.80 [M+1] (Figure 41).
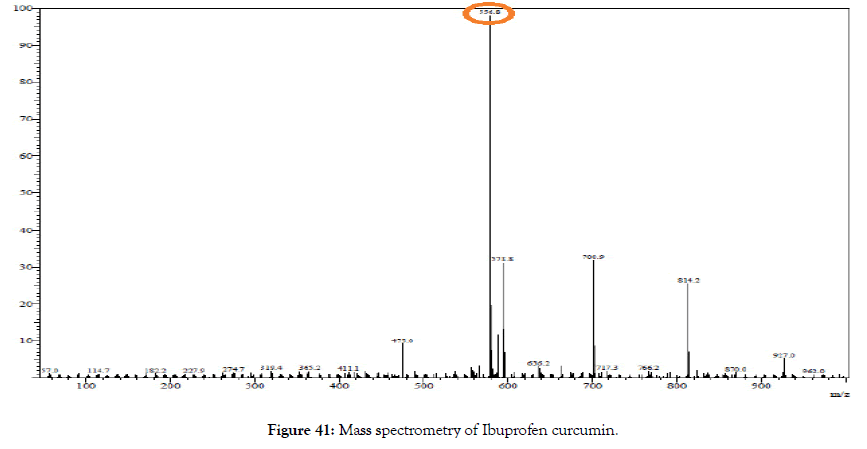
Figure 41. Mass spectrometry of Ibuprofen curcumin.
Figure 42 and Table 2 were shown that the absorbance of curcumin derivatives. The highest and maximum absorption was syringaldehyde curcumin by introducing the methoxy group (-OCH3) which shifted to the red wavelength (429 nm) compared to curcumin and others derivatives (Figure 43).
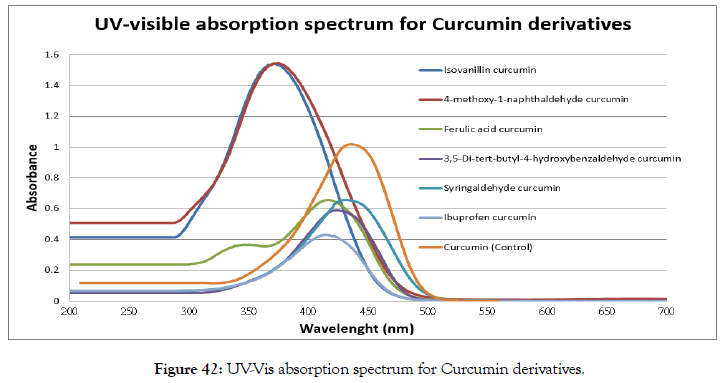
Figure 42. UV-Vis absorption spectrum for Curcumin derivatives.
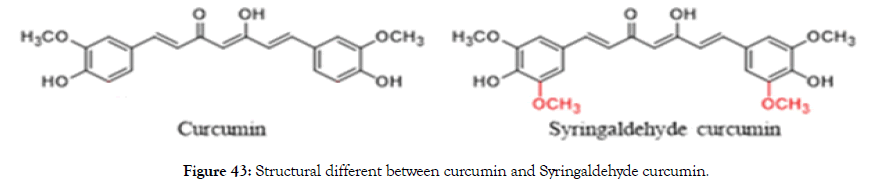
Figure 43. Structural different between curcumin and Syringaldehyde curcumin.
| Curcumin derivatives | Maximum absorption |
|---|---|
| Isovanillin curcumin | 368 nm (max), Broad peak from 285-485 nm |
| 4-Methoxy-1-naphthaldehyde curcumin | 373 nm (max), Broad peak from 282-499 nm |
| Ferulic acid curcumin | 412 nm (max), 333 nm (small peak) |
| 3,5-Di-tert-butyl-4-hydroxybenzaldehyde curcumin | 422 nm (max) |
| Syringaldehyde curcumin | 429 nm (max) |
| Ibuprofen curcumin | 411 nm (max) |
| Curcumin (Control) | 425 nm (max) |
Table 2: Maximum absorption for curcumin derivatives.
Curcumin derivatives were synthesized successfully including ferulic acid curcumin, isovanillin curcumin, 4-methoxy-1-naphthaldehyde curcumin, 3,5-di-tert-butyl-4-hydroxybenzaldehyde curcumin, syringaldehyde curcumin and ibuprofen curcumin. However, the biological activities of these curcumin derivatives would be assessed in our further investigations.
This work was supported by Innovation and Technology Fund of Shenzhen (JCYJ20170307165459562) and Science and Technology Project of Guangdong Province (2017B090911012). We expressed thanks to Prof. Hung-Kay LEE provided his laboratory space for synthesizing curcumin derivatives.
Citation: Law S, Lo C, Han J, Yang F, Leung AW, Xu C (2020) Design, Synthesis and Characterization of Novel Curcumin Derivatives. Nat Prod Chem Res. 8:367. DOI: 10.35248/2329-6836.20.8.367
Received: 19-Dec-2019 Published: 08-Jan-2020, DOI: 10.35248/2329-6836.20.8.367
Copyright: © 2020 Law S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : -