Research Article - (2024) Volume 15, Issue 9
Natural history and Pre-diabetes – Type-2 diabetes (T2DM) is a chronic disease with various metabolic and hormonal disorders derived from genetic and environmental factors that compromise organs and functional systems. Prevention must be initiated in the pre-diabetes phase aimed at high-risk individuals using aggressive measures to reduce excessive body fat with a concerted effort to improve tissue insulin resistance and to protect residual beta-cell insulin secretion. Healthy nutritional adjustments must include low-calorie and low-simple carbohydrate diets with preference for fresh fruits, vegetables, whole grains, more nuts and vegetable oils, lean meats and fish. Less consumption of animal-derived fat, whole-milk dairy products and red meat, and avoidance of sugared beverages. Regular exercises and daily physical activities are recommended. Pharmacotherapy with either metformin and/or pioglitazone is indicated in pre-diabetes individuals with signs of deterioration in glycemic control.
Early T2DM - Once the diagnosis is confirmed, the main goal of therapy shifts and control of hyperglycemia to avoid hospitalization for acid-base and hydro-electrolytic disturbances, acute infections and/or coronary events becomes a top priority. A short trial of insulin therapy or sulfonylurea may be warranted, although these should be rapidly substituted for SGLT- 2i agents with/without GLP-1 RA. A thorough evaluation of glucosedependent microvascular complications such as retinopathy, nephropathy and neuropathies are mandatory. Measures instituted in pre-diabetes must remain in place and, together with new medications; therapeutic objectives are more likely to be achieved.
Late T2DM - As diabetes progresses, efforts to reduce the risk for the development of cardiovascular and renal complications take priority. Dietary recommendations, tailored exercise programs, physical therapy, smoking cessation and promotion of a healthier lifestyle become necessary. A review of anti-atherogenic medications must be implemented. Ultimate objectives in advanced T2DM are to provide quality of life and to help reduce precocious mortality with an extended healthier survival.
Pre-diabetes; Nutritional assessment; Type 2 diabetes; Anti-hyperglycemic agents; Multi-drug therapy; Cardiovascular prevention; Diabetes kidney disease; Risk management.
T2DM: Type-2 diabetes mellitus; OGTT: Oral Glucose Tolerance Test; HbA1c: Hemoglobin A1c; IFG: Impaired Fasting Glucose;IGT: Impaired Glucose Tolerance; FH+: Positive Family History of Diabetes; BMI: Body Mass Index; TNF-alpha: Tumor Necrosis Factor – alpha; WHO: World Health Organization; SGLT-2i: Sodium-Glucose Co-Transporter 2 Inhibitors; GLP-1 RA: Glucagon-Like Peptide 1 Receptor Analogues; DPP- 4i: Dipeptidyl-Peptidase 4 Inhibitors; NHE-1 & NHE-3: Sodium Hydrogen Exchanger 1 & 3.
Diabetes mellitus is a common, worldwide prevalent chronic disease that merits the attention of most health professionals involved in medical practice. Type 1 diabetes mellitus (T1DM) affects primarily young individuals and is characteristically an auto-immune disorder with sudden and irreversible loss of the pancreatic beta-cell insulin secretory capacity. In contrast, type 2 diabetes mellitus (T2DM), essentially a disease of adults, develops gradually and is secondary to a combination of resistance to the action of insulin with progressive loss of pancreatic insulin secretion. In this mini-review we will focus on the medical management of T2DM, which has improved remarkably over the last few decades. Several novel anti-hyperglycemic pharmacologic agents were introduced and, perhaps of greater importance, the emphasis on the main goals of therapy in T2DM patients has shifted slightly. While a decrease in blood glucose levels remains essential, a concomitant effort to control body weight and body fat excess has become a top priority [1,2]. Additionally, considering that cardiovascular and renal diseases are responsible for the increased morbidity and high mortality rates in T2DM [3,4], therapeutic strategies that reduce the burden of these complications and lead to better clinical outcomes are now recommended in early stages of the disease. As a result, some recent analyses and surveys have indicated that there is a trend towards longer survival with less morbidity in T2DM patients [5,6]. Despite substantial improvements however, residual risks persist, and new challenges have emerged. There is still need for more efficient approaches that can halt the rate of progression of the disease to avoid complete and irreversible loss of pancreatic beta-cell function. Likewise, finding the “right timing” to intervene with the “right medications” before reaching an evolutionary point of “no-return”, specifically with regards to the development of micro- and macro-vascular complications is not yet entirely clear. Based on scientific evidence, in this review we propose the implementation of a few reasonable strategies and therapeutic alternatives that are intended to provide superior clinical benefits in T2DM patients (Table 1).
| Type 2 Diabetes | Recommendations |
|---|---|
| Definition | T2DM is a chronic disease with various metabolic and hormonal disorders derived from a combination of genetic and environmental factors that compromise several organs and functional systems over the years |
| Pre-Diabetes | Prevention and treatment must be initiated in the pre-diabetes phase aimed at high-risk individuals with an aggressive management of excessive body fat and a concerted effort to improve tissue insulin resistance and to protect the residual beta-cell insulin secretion |
| Nutritional Adjustments | Healthy nutritional adjustments must include low-calorie and low-simple carbohydrate diets with preference for fresh fruits, vegetables, whole grains, more nuts and vegetable oils and lean meats and fish. Less consumption of animal-derived fat, whole-milk dairy products and red meat, and avoidance of sugared beverages |
| Physical Activity & Pharmaco Prevention | Regular exercises and more daily physical activities are recommended. In pre-diabetes individuals who show signs of deterioration in glucose intolerance, pharmaco prevention with either metformin and/or pioglitazone is indicated |
| Early Diabetes | Once the diagnosis of T2DM is confirmed, the main goal of therapy shifts and control of hyperglycemia to avoid hospitalization for acid-base and hydro-electrolytic disturbances, acute infections and/or coronary events becomes a top priority. A short trial of insulin therapy or sulfonylurea may be warranted, although it is highly recommended that these should be rapidly substituted for SGLT-2i agents with/without GLP-1 RA |
| Diagnosis & Evaluation | At diagnosis, a thorough evaluation of glucose-dependent microvascular complications such as retinopathy, nephropathy and neuropathies are mandatory. Measures instituted during the pre-diabetes stage should remain in place, which in conjunction with the new medications, are more likely to achieve therapeutic objectives |
| Late Diabetes | As diabetes progresses, efforts to reduce the risk for the development and advancement of cardiovascular and renal complications take priority. Dietary recommendations, tailored exercise programs, physical therapy, smoking cessation and the promotion of a healthier lifestyle become necessary. Review of cardiac and reno-protective medications in use must be implemented. The ultimate objectives in advanced T2DM are to provide quality of life and to help reduce precocious mortality with an extended healthier survival |
Table 1: Summary of current risk management strategies in Type 2 Diabetes.
Natural History of T2DM
In order to address the risk management of T2DM patients, it is first necessary to revisit the natural history of the disease. T2DM is diagnosed when fasting plasma glucose reaches 126 g/dl [7.0 mmol/l] or is >200 mg/dl [11.1 mmol/l] 2 hours after an oral glucose load or any meal. The diagnosis can also be made with a Hemoglobin A1c [HbA1c] value equal to or, above 6.5% [47.0 mmol/mol], and it is always prudent to have at least two of these confirmatory tests [2]. These values are somewhat arbitrary and were empirically established with the intent to catch early the appearance of micro-vascular disease in individuals at risk for developing T2DM [7]. It is well known that during the 5 to 10 years preceding the diagnosis of T2DM, individuals at risk already show signs of high susceptibility for the disease. These are categorized as Impaired Fasting Glucose [IFG] and/or Impaired Glucose Tolerance [IGT]. Fasting plasma glucose levels between 100 and 125 mg/dl [5.5-6.9 mmol/l] characterize IFG and values between 140-199 mg/dl [8.0-11.0 mmol/l] in the 2- hour following an oral glucose tolerance test [OGTT] are diagnostic of IGT. At this stage of pre-diabetes, HbA1c values are between 5.7-6.4% [39.0-46.0 mmol/mol], which confirms that the individual has IFG, IGT or both. This is a critically important stage along the disease process and represents the best window of opportunity to intervene with nutritional counseling, lifestyle modifications and pharmacologic agents. The principal objective at this pre-disease stage is to attempt to postpone the appearance of hyperglycemia. Data show that in pre-diabetes beta-cell reserve is severely compromised, I.e., the ability to secrete insulin after a glycemic stimulus is decreased by 60-80% [8]. Yet, circulating insulin concentration tends to be elevated, reflecting increased beta-cell secretion in response to high demands imposed by resistance to the biological action of insulin, primarily at the liver, skeletal muscle and adipose tissue. In fact, the resultant hyperinsulinemia is the reason why fasting blood glucose and postprandial glycemic excursions are maintained within normal range in the pre-diabetes phase. Insulin resistance, both at the cellular and molecular level, is believed to be a fundamental hormonal-metabolic defect that triggers augmented beta-cell insulin response and the eventual exhaustion of pancreatic secretory capacity [9].
Tissue insulin resistance is a condition partially inherited and partially acquired during a lifetime. Numerous candidate genes responsible for the initial disturbances in intra-cellular insulin molecular signaling pathways in various tissues have been identified [10]. The inheritance of impaired insulin signaling in muscle and adipose tissue, as well as in hepatocytes is likely to be secondary to multiple genetic defects. Of clinical relevance, however, is the fact that these genes run in families, especially in first-degree relatives of T2DM patients, who are highly susceptible for developing the disease during their adult life. It has been estimated that a maternal positive family history [FH+] confers a relative risk of 2.0 to 3.4 and, when maternal and paternal FH+ are present the risk is higher, somewhere between 2.6 and 6.1. A paternal FH+ is associated with a relative risk for developing T2DM of 1.4 to 3.5 and FH+ in siblings the risk is near 1.8 [11]. These are the people of greatest interest in any program designed to reduce the conversion from pre-diabetes to diabetes. The risk grows exponentially when first-degree relatives of T2DM patients also have body weight gain with excessive fat accumulation. Overweight and obese individuals, identified by a classification based on body mass index [BMI = Weight in kilograms divided by the square of the height in centimeters] [12], carry an additional acquired form of tissue insulin resistance. The combination of inherited plus acquired tissue insulin resistance in obese first-degree relatives of T2DM patients is accompanied by an exaggerated and continuous demand for beta-cell insulin secretion. An abnormal distribution of fat and its derivatives in various tissues over time is thought to contribute to the blockade of the insulin signaling cascade of events in various tissues. Thus, the inability to advance the molecular messaging of insulin, which is inherited, is further compromised by the accumulation of these fat derivatives in cells [13]. Moreover, most obese individuals tend to deposit excessive fat over time in the abdominal viscera and in organs such as muscle, liver, pancreas, heart, etc., the so-called “ectopic fat”. These adipocytes release pro-inflammatory “adipo-cytokines’, like TNF-alpha, interleukins and others [14] that reach insulin-dependent cells and contribute further to inhibiting intra-cellular metabolic pathways. Consequently, the normal insulin-mediated biochemical and metabolic processes, including glucose transport from the extra- to the intra-cellular space, glycogen synthesis, glycolysis, gluconeogenesis, etc. become impaired, a condition referred to as tissue insulin resistance.
In addition to genetic factors, many environmental factors are known to negatively interfere with the hormonal and metabolic processes and thus, facilitate the progression of T2DM in the adult life. Although the accumulation of excess body fat over time is also thought to have inherited traits, voluntary long-standing positive caloric imbalance is largely responsible for the expansion of the adipose tissue. In modern times, there is a strong universal tendency for the consumption of caloric dense, industrialized, processed food and beverages rich in refined, simple sugars. These are easily accessible, relatively inexpensive and convenient to most hard-working families. Considering that the insulin reserve of the pancreas in susceptible individuals is scarce and, since the ingestion of large amounts of nutrients in general, and more specifically the intake of simple sugars represents a potent stimulus to insulin secretion, it is not surprising that these dietary indiscretions accelerate the loss of insulin reserves. Furthermore, the lack thereof or a very low level of physical activity in most individuals at risk leads to sedentary lifestyle, which also contributes to the development of T2DM. It has been shown that physical activity and exercise training stimulate glucose utilization in skeletal muscle via insulin-independent mechanisms. The resultant muscle growth and hypertrophic tissue, therefore, becomes very sensitive to insulin action [15]. The opposite is true for sedentary people in whom insulin-dependent muscle glucose metabolism is resistant. It is of paramount importance to recognize that environmental factors play a critical role in the pathogeneses of T2DM and that, unlike genetics, they can be modified.
The natural history of the disease indicates that to overcome the state of insulin resistance, the secretion of insulin by the pancreas must be continuously stimulated. Eventually, the enhancement of insulin secretion leads to exhaustion and complete beta-cell failure. Pre-diabetes individuals, having IFG, IGT or both, have near-maximum tissue insulin resistance with compensatory insulin over secretion. The resultant hyperinsulinemia can maintain the blood glucose levels within an acceptable range. While elevated plasma insulin concentration overcomes the hepatic and peripheral insulin resistance, near-normoglycemia is maintained and the effects of glucotoxicity are avoided. However, as the pancreatic insulin secretion becomes unable to meet increasing demands, postprandial and fasting hyperglycemia develop. The oxidative stress induced by persistent hyperglycemia represents an important factor that contributes to the deterioration of pancreatic insulin secretory capacity and worsens tissue insulin action even further. At this point, very high plasma insulin levels are reached but can no longer break through various blockades in the metabolic pathways. As a result, the state of insulin resistant prevails and persists. Hence, when reserves of insulin in the pancreatic beta-cells achieve a critical low level, circulating insulin begins to fall and blood glucose rises. The diagnosis of T2DM is then established and the condition of hyperglycemia, which inhibits insulin secretion and worsens tissue insulin resistance even, further, gives rise to a series of medical complications.
Acute hyperglycemia is often associated with fluid, electrolytes disturbances and acid-base imbalance, which can be severe and lead to hospitalization. High blood sugar exerts toxic effects on capillaries and the microcirculation, alterations which initially become more apparent in retinal vessels and renal glomeruli. Peripheral nerves, mainly those long ones that extend into lower extremities, are functionally compromised by sustained elevations in blood glucose. There is also evidence that in the long run, hyperglycemia, albeit to a minor extent, affects the cardiovascular system. Together with other known risk factors, these manifest early as endothelial dysfunction, which advances to coronary artery, cerebral and peripheral vascular diseases in T2DM. Superimposed on hyperglycemia, traditional risks include obesity with excess visceral and body fat, arterial hypertension and dyslipidemias, all of which accelerate the process of atherosclerosis. Therefore, in view of this diversity of clinical and metabolic complications that tend to accompany T2DM patients, different priorities with specific therapeutic objectives must be established at various stages during the natural history of the disease. Thus, a stepwise approach with specific management strategies divided into three distinct phases of diabetes has been proposed: i) Pre-Diabetes ii) Early, Newly Diagnosed Diabetes, and iii) Late, Advanced Stage of T2DM.
The Pre-Diabetes Phase
The identification of pre-diabetes individuals is the first step to implementing an efficacious diabetes preventive program. People who are overweight or obese and who have a FH+ of diabetes, especially in a first-degree relative, are considered at very high risk for developing diabetes. The presence of a FH+ of diabetes is crucial since having obesity by itself does not necessarily lead to diabetes. Many obese people are considered “metabolically” normal and are thus, protected from developing diabetes and related diseases [16]. Even though accumulation of excessive body fat is an essential contributor to the development of T2DM, only those who carry a susceptible genetic profile, i.e., inferred in first degree relatives, represent the “high-risk” group. This is critical, but resources are sparse, inasmuch as obesity is a chronic condition that is associated with much co-morbidity. Hence, the identification of high-risk individuals among the obese people is very important in order to effectively act to delay the appearance of hyperglycemia. In this selective group, aggressive implementation of preventive measures is more likely to yield results and spare valuable resources. Although recommendations for general screening of all obese individuals have been proposed, first-degree relatives of T2DM subjects have the most to gain from prevention strategies [17]. Needless to say, obesity is linked to numerous functional and esthetic problems that merit specific but a different type of medical attention. It is also important to include women with gestational diabetes in this “high-risk” group. These women should also be managed in the same way as any individual with pre-diabetes. In this regard, immediate action should be taken in the postpartum period and during lactation in all women with the diagnosis of gestational diabetes.
To establish that an individual has pre-diabetes requires the measurement of plasma glucose in the fasting state and/or after an OGTT or else a HbA1c determination. Once the status of pre-diabetes is confirmed, the main objectives of the interventions at this very early stage are: i) to delay and prevent the appearance of hyperglycemia and, ii) to reduce the demand on beta-cell pancreatic insulin secretion. To reach these two goals, manipulations in the ingestion of calories and nutrients that help to reduce the excessive body fat and body weight, as well as regular aerobic and anaerobic exercises in a training program must be implemented as an initial step. Body weight and fat loss and the control of obesity in people with pre-diabetes is of highest priority. After a few months of intense dietary and lifestyle changes, the use of medications known to protect pancreatic beta-cells insulin secretion must be considered. With regards to nutritional recommendations, caloric restriction with an intake of ~500 calories less every day is a good start for an average person. As a rule, incentives to consume “less quantity”, as opposed to specifying the “quality” of the ingested foodstuff are more effective. There are numerous studies indicating that it makes little to no difference whether the restricted caloric source derives from carbohydrates, fat or a combination [18-20]. Thus, to succeed in achieving low-calorie diets it is necessary to limit portion sizes and to avoid “plate refills” in daily meals. Two novel strategies designed to aid in the challenge of inducing sustained body weight loss have been recently introduced, namely “intermittent fasting” [21] and time-restricted eating [22]. Intermittent fasting has been associated with weight loss that appears to be well-tolerated and may be especially useful in some pre-operative conditions. Time-restricted eating, to be applied only a few days every week, also has been shown to induce weight loss with some metabolic benefits in a few small, short-term studies [23,24]. Positive fIndings with these fasting strategies have been reported to be due to good adherence. Body weight changes and metabolic improvements are attributed, at least in part, to the fact that periods of prolonged fasting extend the catabolic phase of the normal circadian rhythm.
One additional nutritional adjustment that favours the loss of body weight and, simultaneously helps to preserve beta-cell function is a reduction in the daily consumption of simple carbohydrates, i.e., refined sugars [25]. This recommendation is strongly supported and disseminated by the World Health Organization (WHO) that has proposed to limit the ingestion of simple sugars to a total daily maximum of 50 grams (200 calories). The official WHO statement [26] mentions the risk of developing diabetes and dental caries in youth who consume an exaggerated amount of “free sugar” added to beverages and food products. The statement also provides various low-sugar food and beverages alternatives. It is important to mention that the WHO recommendation for “low-sugar diets” does not imply that refined sugar should be substituted for non-caloric sweeteners. In the statement there is a compelling incentive, instead, for people to give preference for more naturally sweet products, such as fresh fruits, non-sugared juices, vegetables, whole grains, etc. The utilization of non-caloric sweeteners is a personal decision and may be safe, as long as the choice among the available sweeteners in the market meets minimal scientific standards, is consumed in very low quantities and is approved by regulatory organs. It is imperative, however, to recognize that a recommendation for “low simple carbohydrate diets” does not presuppose “low carbohydrate diets”. A variety of complex carbohydrates and fibre-rich food products are available that can easily fulfil the requirements of a typical Western diet containing 45-55% percent of carbohydrates.
Adherence to these nutritional recommendations is frequently accompanied by 5-10% body weight loss over 6-12 months in the majority of obese people. This seems to be sufficient to improve a few but essential metabolic and hormonal derangements, at least temporarily. Of interest, after weight loss, a “legacy period” with persistent cardio-metabolic benefits has been described, which happens whether body weight is regained partially or restored completely [27]. Thus, in obese pre-diabetes individuals, any form of manipulation in the intake of calories and nutrients that induces loss of body weight with reduction in the excess of body fat is worth a trial. Even a small amount of sustained weight loss has been shown to improve tissue insulin resistance and to postpone beta-cell exhaustion, thus avoiding hyperglycemia. As a result, the conversion from the pre-diabetes phase to frank diabetes is retarded. This was well documented in the work of Tuomilehto, and collaborators published in 2001 [28], which demonstrated that an average body weight loss of 3.0-4.2 kg [~5%] over three years in obese pre-diabetes individuals following a low-calorie diet led to a decrease in the annual conversion rate to 2.8% versus 6.0% in the control group. Identical results were also reported in a much larger study, the Diabetes Prevention Trial [29], in which a group of 3,234 obese people on a caloric restriction diet had a mean loss of body weight of ~7% [5-7 kg] after three years. A reduction in the annual conversion rate from pre-diabetes to diabetes of ~50%, i.e., from 11.0% in the control group down to 4.8% in the intervention group, was then registered. These were among the first observations to confirm that body weight loss, regardless of the means, is accompanied by a decline in the rate of appearance of T2DM in high-risk populations. Of additional interest, the appearance and progression of microvascular diseases and cardiovascular and renal complications were also postponed.
Based on these scientific reports, it is recommended that individuals in the pre-diabetes phase adhere to low-calorie diets with limited intake of simple sugars. Instead, the incentive should be on the intake of fresh fruits and fibre-rich complex carbohydrates. Moreover, the substitution of most saturated animal fat and of processed, industrialized food products for mono- and poly-unsaturated fat found in vegetables and fish is strongly suggested. In this regard, more frequent consumption of low-fat dairy and “lean cuts”, olive oil and nuts, as well as seafood products is recommended. People following DASH (Dietary Approach to Stop Hypertension) and Mediterranean-type diets have shown significant less cardiovascular events and mortality [30,31]. In one study there was a concomitant ~60% decrease in the annual conversion rate of pre-diabetes to diabetes [31]. Thus, in complying with these nutritional adjustments pre-diabetes individuals are expected have a delay in the development of hyperglycemia, better protection of beta-cell function and a reduction in cardiovascular risk.
It is necessary to briefly discuss weight loss with the use of adjunct pharmacologic agents and of bariatric procedures in the management of extremely obese people with T2DM. As mentioned above, 5-10% of body weight loss after a period of approximately one year is accompanied by a few cardio-metabolic benefits [27,32]. Nutritional manipulations combined with pharmacologic agents and/or bariatric procedures may be necessary to achieve these goals in specific circumstances, although loss between 25-40% of the original body weight is the rule. There are currently numerous pharmacologic agents approved for weight loss that can be used in obese non-diabetic patients, a strategy which could benefit pre-diabetes individuals interested in supplementing nutritional therapy to achieve adequate weight reduction. In those with BMI above 35 kg/m2 or above 30 kg/m2 with co-morbidities, including pre-diabetes, bariatric procedures are indicated. In these individuals, achieving greater weight loss is known to be associated with diabetes prevention and substantial cardio-metabolic benefits [33-35]. Keep in mind, however, that in addition to fulfilling the needs for cardio-metabolic disease prevention, such large reductions in body weight also provide other medical benefits. The typical respiratory restrictions and discomfort seen in these extreme obese patients are alleviated. Also, they experience improvements in mobility dysfunction with less osteo-articular limitations and, in some cases, there are obvious aesthetic gains. Further detailed description of the “pros and cons” of bariatric procedures in the management of obesity is beyond the scope of this review.
Physical activity
The stimulation of physical activity is a critical aspect of a series of lifestyle modifications that must be addressed in individuals with pre-diabetes in an effort to improve the chances of succeeding in diabetes prevention. Sedentary lifestyle and rare practice of regular exercises are known to be associated with muscle insulin resistance and thus, facilitate the conversion from pre-diabetes to diabetes. There is good evidence that training with repetitive muscle contractions leads to improved insulin sensitivity in skeletal muscle, which further contributes to delaying beta-cell failure and hyperglycemia [15,36]. Modern life relies heavily on “automatism” with a tendency to make the daily routine less physically demanding. Therefore, to provide motivation to sedentary obese pre-diabetes individuals in order to enhance everyday physical movements, such as creating habits of walking short distances, occasionally using staircases instead of elevators, standing and frequently circulating at home and in the workplace, as opposed to sitting all the time is fundamental. In addition, people at risk must be instructed to find time and exercise regularly including aerobics, such as walking, jogging, swimming, etc., alternating with anaerobic exercises. It has been demonstrated that cardio-metabolic benefits can be obtained with at least 30 minutes of aerobic and anaerobic exercises three to five times every week [37,38].
Preventive pharmacologic interventions
Despite adherence to nutritional recommendations and lifestyle changes, pharmacologic interventions often become necessary to help further delay the conversion of pre-diabetes to diabetes. As plasma glucose levels rise or the HbA1c values increase, even if they remain within the pre-diabetes range, the addition of either metformin or pioglitazone to “diet and exercise” plans is warranted. Metformin, at the dose of 500 mg up to 1000 mg twice daily has been demonstrated to prevent T2DM [29]. Metformin inhibits endogenous release of glucose and attenuates the demand for insulin secretion, which tends to extend the pancreatic beta-cell insulin reserve. Metformin has been associated with occasional gastrointestinal discomfort and diarrhea and, rarely with hypoglycemia, most frequently in patients during prolonged fasting or alcohol binge. The use of pioglitazone, at the dose of 15, 30 and up to 45 mg daily, as a second option or as an add-on to metformin is also very effective in preventing T2DM. In a clinical trial, treatment with pioglitazone was able to delay the appearance of hyperglycemia and retard the conversion to T2DM in pre-diabetes individuals [39]. Peripheral edema and weight gain are common side-effects, and the development of osteopenia/osteoporosis has been described in some patients taking pioglitazone. It is contraindicated in advanced congestive heart failure, as pulmonary edema may occur. The mechanism underlying the preventive effects of pioglitazone, a PPAR-gamma agonist, is linked to the sensitization of insulin action in skeletal muscle, adipose tissues and liver. As a result, peripheral utilization of glucose is enhanced, and with attenuation of hepatic gluconeogenesis, the release of glucose into the circulation diminishes. As a consequence, tissue insulin resistance is abated, circulating glucose levels are reduced and beta-cell demand for insulin secretion decreases. Because the mechanisms of action of metformin and pioglitazone do not overlap entirely, these drugs used together provide additive effects. The initial management of pre-diabetes individuals with nutritional adjustments and regular physical activity thus, may require the addition of one or both of these two medications in order to retard the disease advancement. This decision relies essentially on close monitoring and signs of worsening glycemic indices over time. Eventually, however, all preventive measures tend to become ineffective and with progressive loss of beta-cell insulin reserves, tissue insulin resistance prevails. At this point, hyperglycemia becomes evident and the conversion of pre-diabetes to T2DM occurs.
Early Phase of T2DM
The clinical diagnosis of T2DM is established when fasting plasma glucose reaches a value equal to or above 126 mg/dl [7.0 mmol/l] and/or a post-OGTT or post-prandial value above 200 mg/dl [11.0 mmol/l] is documented. The diagnosis of T2DM can also be made with HbA1c values equal to or higher than 6.5%. Once the diagnosis is confirmed, the main objective in the management at this early phase of the disease shifts slightly and turns towards the control of hyperglycemia. In addition to maintaining all the measures already implemented in the pre-diabetes stage to reduce and maintain adequate body weight, improve tissue insulin resistance and preserve beta-cell secretory reserves, effective anti-hyperglycemic therapies must be undertaken. The reason behind this aggressive attempt at glycemic control relates primarily to the avoidance of acute complications. These include dehydration, acid-base and electrolyte disturbances, visual symptoms, as well as sensory and painful neuropathies. There is also an increased risk of infections and of acute coronary events, all of which might require hospitalization. These are secondary to hyperglycemia and may evolve into life-threatening situations. Immediately upon the diagnosis, some might recommend that in mild cases of asymptomatic hyperglycemia and also in non-hospitalized patients, a limited trial of a sulfonylurea is warranted. This is because quick and efficient secretion of insulin stimulated by sulfonylureas is often enough to lower blood glucose. In contrast, however, in those patients in whom hyperglycemia develops rather rapidly and becomes symptomatic, treatment with insulin injections may be the best choice. This is especially true for all seriously ill and hospitalized T2DM patients, even if the decision to start insulin therapy is temporary.
In general, sick diabetes patients who are admitted to the hospital with uncontrolled hyperglycemia, require insulin therapy. Initially an insulin sliding scale using subcutaneous short-acting insulin injections every 4-6 hours is necessary. After a few days into this “insulin scale” regimen, a single daily dose of long-acting basal insulin must be added. This insulin combination regimen is intended to achieve and maintain plasma glucose levels between 100-150 mg/dl, as long as the patient is hospitalized [40,41]. Once clinical conditions become more stable and glycemic levels are under control, serious consideration must be given to discontinuation of the insulin injections. Prior to the hospital discharge a diabetes education program should be initiated and information about self-management and resources available to patients and family members must be provided. In addition, a careful review of dietary choices, lifestyle changes and a discussion about the transition from the insulin regimen to other anti-hyperglycemic agents is also needed. In view of the wide range of options with many efficacious drugs currently available to manage T2DM patients, implementation of an insulin therapy regimen to treat recently diagnosed T2DM in the outpatient setting is usually not a first choice. Upon hospital discharge, follow-up ambulatory visits are scheduled within 1-2 weeks to secure treatment continuity and focus on the primary goal of glycemic control. Most approved drugs used in early diabetes management are known to minimize the acute complications of hyperglycemia and to protect islet-cell function. Unlike insulin therapy, most of these anti-hyperglycemic agents contribute to weight loss, which is a critical part of overall diabetes treatment.
In most ambulatory T2DM patients in stable clinical conditions who are being treated with sulfonylureas, whether near-normoglycemia has been reached or not, a switch to other anti-hyperglycemic agents is often desirable. Even though sulfonylureas induce decreases in blood glucose within days or weeks, it is important to realize that sulfonylureas are potent glucose-independent insulin secretagogues and the risk of developing hypoglycemia is always high. There are also reports that sulfonylureas may accelerate beta-cell exhaustion over time [42] and the resultant hyperinsulinemia contributes to weight gain, which further complicates the management of diabetes. Although useful for a short period of time, there is no clear advantage of a particular sulfonylurea drug over any of the others. It seems prudent to consider, then, results showing that all sulfonylureas lack effectiveness in maintaining lower blood glucose and adequate HbA1c values after one year of treatment [43]. Thus, it is highly recommended that sulfonylureas be used only in newly diagnosed T2DM patients and for a short period, not to exceed 6-12 months. Once discontinued, treatment with sulfonylureas is substituted for safer and more effective anti-diabetic agents. Many of recently introduced anti-diabetic agents not only maintain adequate glycemic control for longer periods of time but also help to reduce body weight, preserve beta-cell insulin secretion, improve tissue insulin resistance and prevent complications.
Sodium-glucose co-transporter 2 inhibitors (SGLT-2i) are part of a novel class of anti-diabetic agents that inhibit proximal tubular glucose reabsorption to induce glycosuria. As a consequence, blood glucose levels decrease rapidly, and the development of complications due to sustained hyperglycemia is halted. Treatment with SGLT-2i is also associated with body weight loss and it has thus, become an excellent choice in these early stages of diabetes [44-47]. Several SGLT-2i drugs are currently approved by the U.S. Food and Drug Administration to be used alone or in combination therapy in T2DM patients [48]. The reasons behind the preferential utilization of SGLT-2i rests on solid clinical data indicating that, in addition to effectively controlling hyperglycemia, reducing systemic blood pressure and promoting mild but sustained weight loss, these agents decrease major cardiovascular events [49-51] and slow the progression of chronic kidney disease in T2DM patients [52-54]. Nowadays, the introduction of SGLT-2i agents early in the management of T2DM, who are already on metformin and/or pioglitazone therapy, has become common practice [47]. With the growing use of these drugs, however, it is important to remember that treatment with SGLT-2i may be accompanied by genital mycotic and urinary tract infections at times and, that there is a high risk of dehydration with hypotension, especially in elderly patients and in those using loop diuretics. Rarely, an euglycemic form of keto-acidosis [55] can develop, which is more likely to occur in critically ill T2DM or in insulin-dependent T1DM patients [56].
Empagliflozin at the initial dose of 10 mg and up to 25 mg daily is approved to be used in the treatment of T2DM to achieve and maintain near normoglycemia. A decrease in blood pressure with simultaneous reductions in body weight is expected, as long as the drug is well-tolerated. According to recent clinical trials [49,54], long-term cardiovascular and renal benefits are also anticipated in most patients. Another treatment option is Dapagliflozin at the initial dose of 5 and up to 10 mg daily, which provides identical blood glucose lowering, blood pressure and body weight loss, as well as comparable cardiovascular and renal benefits [50,53]. Canagliflozin at the dose of 100 mg up to 300 mg daily is an additional choice for initiating SGLT-2i therapy in T2DM patients. It is also accompanied by comparable anti-diabetic, anti-hypertensive and weight loss benefits, as well as by cardiovascular and renal protective effects [51,52]. Ertugliflozin belongs to the class of SGLT-2i agents and is approved in the U.S. with the same indications for the treatment of T2DM. There are numerous other SGLT2-i drugs available worldwide, and there is a consensus that all clinical and metabolic beneficial effects reported with a few SGLT-2i agents can be generalized, since there are no substantial structural and pharmacodynamic differences between them. The best drug of choice within the class for any given patient, therefore, should be based on individual preferences, accessibility and affordability. The use of SGLT-2i in combination with insulin injections or sulfonylureas increases the risk of hypoglycemia and caution is recommended. A few trials of SGLT-2i therapy that included T1DM patients [57-59] concluded that, under special circumstances and close supervision, these agents can be used to supplement the insulin injections. Whether the benefits outweigh the risks, however, remains to be seen.
GLP-1 receptor analogs (GLP-1 RA) are an efficacious class of anti-diabetic agents that provide adequate glucose-lowering effects and weight loss with significant cardiovascular and renal benefits. The addition of GLP-1-RA agents to the management of T2DM in the early stages represents a viable and reasonable alternative. This is particularly true for obese T2DM patients who have difficulty losing weight despite strict dietary adjustments. GLP-1 RA can be used either alone or combined with SGLT-2i agents in newly diagnosed T2DM. Together with metformin plus pioglitazone dual therapy, already instituted during the pre-diabetes phase, a triple or quadruple therapy may be entertained to neutralize the majority of active pathogenetic factors in early diabetes. The advantages of an initial aggressive multi-pharmacologic therapeutic approach are supported by a few recent observations demonstrating superior long-term clinical benefits in T2DM patients versus other tested regimens [60-62]. The addition of GLP-1 RA to the treatment helps to decrease circulating glucose levels via a physiological stimulation of beta-cell insulin secretion with concomitant suppression of alpha-cell glucagon release. These are glucose-independent actions and are particularly useful in the post-prandial period to attenuate hyperglycemic excursions. The observation that GLP-1RA also reduces fasting blood glucose levels further contributes to better overall glycemic control over time [63]. Moreover, it has been suggested that the insulin secretagogue effects of GLP-1 RA, unlike sulfonylureas, are accompanied by preservation of beta-cell function in vivo [63,64], although most data are extracted from experiments with islet cell cultures and in animal models [65]. One aspect of fundamental importance during GLP-1 RA therapy is body weight loss observed in most patients. The latter is due to slowing of gastric emptying combined with appetite suppression and satiety control, both via a central action on the hypothalamus [63,65].
Several GLP1-RA compounds are currently approved and available on the market and, can be utilized to initiate treatment of T2DM patients [66]. Exenatide is an original molecule extracted from the “Gila Monster” that mimics the human GLP-1 RA, structurally and functionally [67]. At the dose of 5 µg injected subcutaneously twice daily prior to meals, it is capable of reducing extreme postprandial glycemic elevations. Just as effective, Exenatide at the dose of 2 mg injected weekly was developed next. Liraglutide is another efficacious alternative and can be given as once daily injections at the dose of 0.6 mg up to 3.0 mg. Of interest, at the highest dose of 3.0 mg, Liraglutide was shown to induce 5-10% of weight loss in a majority of obese T2DM patients after one year of treatment [33]. GLP-1 RA medications injected weekly can also achieve adequate glycemic control. Dulaglutide at the dose of 0.75 mg up to 4.5 mg weekly injections reduces postprandial glycemic excursions and is associated with body weight loss. Recently, Semaglutide at the dose of 0.25 mg and up to 1.0 mg weekly was introduced to treat hyperglycemia in T2DM patients and, at the highest dose of 2.4 mg weekly, it was demonstrated to induce greater body weight reductions in adult overweight and obese people as compared to other agents in the GLP-1 RA class [68]. Even though less effective, an oral formulation of Semaglutide at the dose of 3, 7 up to 14 mg daily were also introduced and is now available on the U.S. market.
More recently, a novel dual GLP-1/GIP agonist, Terzipatide and a triple GLP-1/GIP/Glucagon agonist, Retatrutide, have been developed and clinically tested in diabetes and obese subjects. Both of these have been associated with superior weight loss as compared to any other GLP-1 RA agent, despite comparable anti-hyperglycemic effects [69,70]. In T2DM patients, the dual agonist Terzipatide at the injectable dose of 2.5 mg weekly up to 15 mg reduces HbA1c by 1.0-1.5% with sustained weight loss of nearly 15% after one year of treatment. In a Phase II clinical trial including obese individuals, Retatrutide injected weekly at the highest dose of 12 mg was accompanied by ~24% body weight loss after 48 weeks of treatment. All of these agents, GLP-1 RA, dual and triple-agonists, categorized as “incretin therapy”, are now indicated early in the management of obese T2DM patients. Here, it is worth mentioning that although treatment with dipeptidyl peptidase-4 (DPP-4) inhibitors is also a form of “incretin therapy”, these agents have weak anti-hyperglycemic effects, induce no significant weight loss and, neither insulin secretion nor resistance improves. Thus, the use of DPP-4 inhibitors is not a first line recommendation in the treatment of T2DM, except perhaps in exceptional circumstances. Consistent with the principal objectives of early diabetes treatment, however, “incretin therapy”, excluding DDP-4 inhibitors, is an excellent choice. In part, this is because a decline in body weight with reductions in excessive body fat is of primary concern and takes precedent over the glycemic control in obese T2DM patients [2]. The rationale behind the shift in priorities is that following weight loss, insulin resistance improves, and blood glucose levels tend to fall, whereas not all anti-hyperglycemic agents are capable of improving insulin action or inducing sustained weight loss.
Prior to introducing “incretin therapy” into the management of T2DM patients, nonetheless, one should keep in mind that GLP-1 RA, as well as dual and triple-agonists, are associated with a few important adverse events and have specific contra-indications [66]. Gastrointestinal symptoms, including nausea, vomiting and diarrhea are frequent in patients on “incretin therapy”, even though they are usually transient and dose dependent. Acute pancreatitis with/out the presence of bile tract stones has been reported to which rapid weight loss may contribute. Patients with a diagnosis of thyroid medullary cancer or MEN-2 Syndrome are not candidates for “incretin therapy”, since thyroid malignancies have been detected in experimental animals exposed to GLP-1 RA agents. These drugs are contraindicated in patients with gastroparesis, recurrent pancreatitis and also should be avoided during pregnancy, lactation and in advanced renal insufficiency. Initiation of “incretin therapy” together with metformin plus pioglitazone is highly recommended, particularly in recently diagnosed obese T2DM patients. This combination of triple therapy has been shown to be very effective in achieving both the objectives of minimizing acute complications of hyperglycemia and slowing the decline in beta cell function [60-62]. As deemed clinically necessary, quadruple therapy with the addition of SGLT-2i agents provides further glucose lowering effects, greater weight loss and a better control of arterial hypertension. The rationale behind an early start with quadruple therapy in obese T2DM patients relies on the fact that there are numerous pathogenic factors contributing to the progression of diabetes. The majority of these must be neutralized early in order to increase the chances of halting the advancement of the disease with less metabolic, cardiovascular and renal complications.
The many factors known to contribute to the pathogenesis of T2DM are still active and ongoing at the initial stage of the disease (Figure 1). To partially restore the metabolic and hormonal imbalances that accompany patients with diabetes, these defects must be corrected. Inasmuch as the ideal “timing” for such aggressive intervention is not yet entirely clear, early implementation of a multi-drug regimen in the management of T2DM is believed to be a good window of opportunity to maximize clinical benefits. Dietary adjustments and lifestyle modifications in combination with multiple pharmacological agents can modify most of these factors and represents the best option to slow the disease progression. This aggressive intervention is capable of controlling abnormally elevated circulating levels of glucose with attenuation of the disturbed lipid metabolism, thus helping to reduce the effects of glucotoxicity and lipotoxicity. Further actions include improvements in tissue insulin resistance and stabilization of the islet-cell function, while partially restoring the normal “gut-pancreas axis”. Body weight loss with reduction in the excessive body fat weakens the release of toxic pro-inflammatory adipocytokines. If started early, this multi-drug medical intervention is likely to have a great impact on the natural history of the disease [27]. Of additional clinical interest, serious undesired events, such as severe hypoglycemia weight gain, etc. are rare with the use of these multiple agents in combination, except when insulin injections and/or sulfonylureas are part of the treatment. Although, the adverse effects and contra-indications, as outlined previously, must be taken into consideration when treatment with metformin, pioglitazone, SGLT-2i and “incretin therapy” is started. As a rule, it is essential to insist on and maintain a hypocaloric diet with restriction of simple sugars and to re-emphasize the absolute need for regular physical activity, as critical components in the early management of T2DM patients.
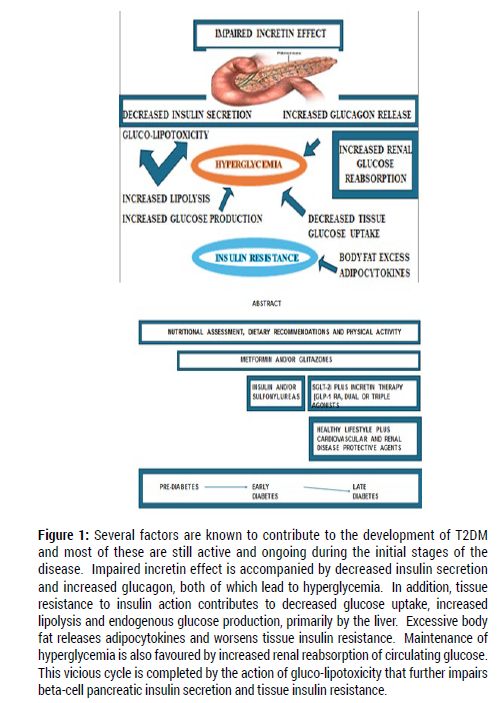
Figure 1: Legend
Late, Advanced Stages of T2DM
Despite aggressive therapeutic interventions patients with T2DM almost invariably advance to later stages of the disease when serious complications tend to occur. The progression of the disease may be either slower or faster, depending upon the effectiveness of and adherence to the therapeutic regimen. There is also an individual, perhaps genetic, component that determines the response to any particular anti-diabetic agent. Moreover, it is all too common for T2DM patients to seek medical attention who are already in advanced stages of the disease and have thus, not had the opportunity to receive initial adequate treatment and counselling. Of practical interest, however, the focus at the late, advanced stages of T2DM change significantly and the primary management goals are to provide better quality of life and help to reduce the precocious mortality and to extend a healthy survival. To this end, it becomes critical to intensify the control of risk factors that predispose to the development and advancement of the atherosclerotic cardiovascular disease and renal complications. This does not imply that efforts to achieve and maintain glycemic control and weight reduction should be abandoned. On the contrary, pre-established nutritional recommendations, lifestyle modifications in conjunction with efficacious anti-obesity and anti-hyperglycemic regimens should be maintained. It is possible that at this advanced stage of the disease nothing else, but insulin therapy must be added to help control hyperglycemia. In addition, T2DM patients with evidence of proliferative retinopathy, micro- and macro-albuminuria with loss of glomerular function and/or serious painful, sensory or autonomic neuropathy will require counselling of Medical Specialists. Medications intended to reduce atherogenic cholesterol particles in the circulation, alpha and beta-adrenergic blockers, anti-coagulants, aspirin and ACE inhibitors or angiotensin receptor blockers, as well as anti-hypertensive agents such as calcium-channel blockers and others must be part of a multi-drug approach. These well-known anti-atherogenic, cardio-protective agents must be initiated, and the dose adjusted individually, as tolerated. Regarding diabetes kidney disease, in addition to rigorous hypertension and blood glucose control, a trial of aldosterone receptor inhibitors, spironolactone or finerenone, may help to slow the progression towards end-stage renal disease [71]. Thus, intense management of atherosclerotic cardiovascular diseases and of chronic renal insufficiency should be given top priority in the late stages of the disease. This may require input from Cardiovascular and Nephrology Specialists, among others. It is important to keep in mind that these conditions are highly prevalent and impact negatively on the quality of life and largely responsible for precocious and increased rates of mortality in T2DM patients.
In the last few years, notable declines in the burden of cardiovascular disease in obese individuals and T2DM patients have been published in association with some specific nutritional and with aggressive weight-loss interventions. Following a Mediterranean-type diet was shown to induce stabilization of body weight, a delay in the conversion from pre-diabetes to diabetes and a decrease in the number of cardiovascular events in aground of “healthy” obese subjects [31]. Mediterranean-type diets consist of limited consumption of simple sugars substituted for fresh fruits and vegetables and, more fish and less red meat and industrialized food products; the intake of animal fat should be largely substituted for vegetable oils, mainly olive oil, and nuts. Of additional interest, these diets have a record of good adherence with better long-term compliance, as compared to other dietary manipulations [72]. Comparable results also have been reported with the “DASH diet” in hypertensive and T2DM patients with chronic kidney disease [73]. DASH diets emphasize low-salt intake and a preference for whole-grains but are otherwise very similar in composition to Mediterranean-type diets. In extremely obese patients with T2DM serious consideration should be given to bariatric procedures, endoscopic or surgical, especially at this advanced stage of the disease. Extensive body weight and fat loss can be achieved rapidly and there is a substantial decline in the risk of cardiovascular death with a potential for diabetes remission [74-76]. As always, it is imperative for patients at this late stage to maintain and pursue appropriate levels of physical activity, including adequate physical therapy. Needless to say, smoking cessation is a “must” and adherence to a healthy lifestyle is mandatory in order to achieve the desired improvements in quality of life and to prolong survival.
One of the greatest recent advances in the pharmacotherapy of T2DM patients was the demonstration in large clinical trials that, in addition to achieving glycemic control and weight reduction, SGLT-2i and GLP-1 RA agents are very effective in reducing cardiovascular and renal endpoints. Empagliflozin given to a group of T2DM patients with evidence of atherosclerotic cardiovascular disease for a period of nearly 4 years was accompanied by ~14% reduction in coronary and cerebrovascular events and by ~35% decrease in hospitalization for congestive heart failure. The study reported 38% less cardiovascular and 32% less total mortality versus a placebo group with comparable glycemic control [49]. Similar results were also obtained with long-term therapies using Canagliflozin [50] and Dapagliflozin [51] in T2DM patients who had varying degrees of atherosclerotic disease. Of special interest, in all clinical trials cardiovascular benefits were detected a few months after the introduction of SGLT-2i treatment. Based on these findings, the addition of SGLT-2i agents to the management of T2DM at any stage and is now universally recommended. Furthermore, and of enormous clinical significance, regimens that include SGLT-2i drugs have been associated with slow progression of diabetes kidney disease and lower mortality rates [52-54]. A delay in reaching end-stage renal disease with decreased requirements for dialysis and/or renal transplantation, as well as a reduction in mortality rates attributed to chronic uremia have also been reported with SGLT-2i therapy in T2DM patients who have loss of glomerular filtration and proteinuria. A list of clinical trials with cardiovascular and renal endpoints during SGLT-2i therapy in T2DM patients is summarized in Table 2.
| EMPA-REG (7,020) [49] | Canvas (10,142) [50] | Declare-TIMI (17,160) [51] | Vertis-CV (8,246) [48] | Credence (4,401) [52] | DAPA-CKD (2,152) [53] | EMPA-Kidney (6,609) [54] | Scored (10,584) [48] | |
|---|---|---|---|---|---|---|---|---|
| Drug | Empagliflozin | Canagliflozin | Dapagliflozin | Ertugliflozin | Canagliflozin | Dapagliflozin | Empagliflozin | Sotagliflozin |
| Age (y) | 63 ± 7 | 63 ± 10 | 64 ± 7 | 64 ± 9 | 64 ± 8 | 64 ± 7 | 67 ± 14 | 69 ± 7 |
| Dosage | 10-25 mg/day | 100-300 mg/day | 10 mg/day | 5-15 mg/day | 100-300 mg/day | 10 mg/day | 10-25 mg/day | 200-400 mg/day |
| HbA1c (%) | 8.1 ± 1.6 | 8.2 ± 1.3 | 8.3 ± 1.5 | 8.2 ± 1.0 | 8.3 ± 1.5 | 8.3 ± 1.2 | 8.2 ± 1.6 | 8.3 ± 1.5 |
| CVD+ | 100% | 65% | 41% | 100% | 50% | 38% | 26% | 50% |
| HF+ | 12% | 11% | 9.9% | 23.4% | 14.8% | 11% | 9% | 31% |
| eGFR ml/min. 1.73m2 | >30 | >30 | >60 | >30 | 30-90 | 25-75 | 20-45 | 25-60 |
| Median F/U (years) | 3.1 | 2.4 | 4.5 | 3.0 | 2.6 | 2.4 | 2.0 | 1.3 |
| Key Findings | Reduction - 14% MACE 46% Renal Composite | Reduction - 14% MACE 40% Renal Composite |
Reduction - 14% MACE 47% Renal Composite |
Reduction- 3% MACE 19% Renal Composite |
Reduction - 20% MACE 30% Renal Composite |
Reduction - 29% MACE 44% Renal Composite |
Reduction - 16% MACE 28% Renal Composite |
Reduction- 23% MACE 29% Renal Composite |
Table 2: List of SGLT-2i Clinical Trials with Cardiovascular and Renal Benefits.
The reasons behind the cardiovascular and renal benefits associated with SGLT-2i therapy are not entirely clear. Some potential mechanisms have been postulated and relate to the fact that SGLT-2i agents are osmotic diuretics, i.e., glucosuretics, and lower arterial blood pressure. These hemodynamic effects tend to decrease both cardiac pre- and post-load and thus, improve cardiac function. It is intriguing, nevertheless, that numerous other diuretics and anti-hypertensive medications have never been reported to induce similar benefits in T2DM patients. Thus, additional factors might be involved, such as the theory implying that the hyperactive sodium-hydrogen exchanger isoform NHE-1 is inhibited by SGLT-2i agents across myocardiocytes in T2DM patients with heart failure. If true, removal of excessive sodium and normalization of hydrogen ion entry corrects intracellular pH, reduces ischemia and fibrosis and facilitates myocardial contraction [77]. It has also been implied that the same SGLT-2i action may also be playing a role in the renal tubules and microcirculation, where the isoform NHE-3 is upregulated and presumably responsible, in part, for sodium retention, glomerular hyperfiltration and mesangial proliferation [77]. Hence, the concomitant inhibition of NHE-3 could explain some of the renal benefits of the SGLT-2i agents. In addition to controlling hyperglycemia and systemic hypertension and, by inducing weight loss, SGLT-2i therapy also interferes with the renal tubular ionic exchange system and reabsorption process. Additionally, a decrease in intra-glomerular pressure due to a combination of afferent vasoconstriction [78] and efferent vasodilation [79,80] has been documented both in type 1 and 2 diabetes patients. Altogether, these intrinsic renal effects are believed to diminish the oxygen demand by reducing the renal energy requirements and thus, contributing to stabilization of kidney disease [81]. Interestingly, the observations that the cardiovascular benefits and reno-preventive effects of SGLT-2i treatment have also been reported in non-diabetic patients and in those with non-diabetic renal disease lend further support to the theory that beneficial mechanisms go beyond glycemic control [82,83].
Incretin therapy also is associated with clinically important cardiovascular and renal benefits in patients with T2DM. In the LEADER trial [Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results] more than 9,000 T2DM patients with a high risk of cardiovascular disease receiving Liraglutide for 3.8 years had a ~13% decrease in a composite endpoint including non-fatal myocardial infarction, non-fatal stroke and cardiovascular death [84]. Comparable results using weekly GLP-1 RA were also observed in clinical trials with Semaglutide (SUSTAIN-6), Dulaglutide (REWIND), Albiglutide (HARMONY) and Exenatide (EXSCEL) [85-88]. It is worth mentioning that a majority of the T2DM patients enrolled in these studies had atherosclerotic cardiovascular disease already established. Therefore, all observations published, except for the REWIND trial [86], reflect essentially secondary, not primary, cardiovascular prevention. Remarkably, evidence of renal protection following treatment with GLP-1 RA in T2DM patients with early signs of diabetic nephropathy has also been reported in numerous independent clinical trials. Short-acting Liraglutide and Lixisenatide as well as Semaglutide, Dulaglutide and Albiglutide weekly injections have all been demonstrated to reduce albuminuria, slow glomerular filtration loss and delay the progression of diabetes kidney disease [89-92]. Much like cardiovascular, the mechanisms underlying renal benefits of GLP-1 RA therapy remain elusive. Glucose lowering and weight reduction certainly contribute, although additional molecular, metabolic and hemodynamic factors are also believed to be involved [63,93]. A list of clinical trials with cardiovascular and renal benefits during GLP-1 RA therapy in T2DM patients is summarized in Table 3.
| Leader (9,340)[84] | Elixa (6,068)[90] | Sustain-6 (3,297)[85] | Exscel (14,752)[88] | Harmony (9,463)[87] | Rewind (9,901)[86] | Pioneer (3,183)[89] | Amplitude-O (4,076)[91] | |
|---|---|---|---|---|---|---|---|---|
| Drug | Liraglutide | Lixisenatide | Semaglutide | Exenatide | Albiglutide | Dulaglutide | Semaglutide ORAL | Efpeglenatide |
| Age (y) | 64 ± 7 | 60 ± 10 | 65 ± 7 | 62 ± 9 | 64 ± 7 | 66 ± 7 | 66 ± 7 | 65 ± 7 |
| SEX M F |
64% 36% |
69% 31% |
61% 39% |
62% 38% |
69% 31% |
54% 46% |
68% 32% |
67% 33% |
| Dosage | 1.8 mg/day | 10 or 20 µg/day | 0.5 or 1.0 mg/week | 2.0 mg/week | 1.5 mg/week | 1.5 mg/week | 14 mg/day | 4.0 or 6.0 mg/week |
| HbA1c | 8.7 ± 1.6% | 7.7 ± 1.3% | 8.7 ± 1.5% | 8.1 ± 1.0% | 8.7 ± 1.5% | 7.3 ± 1.1% | 8.2 ± 1.6% | 8.9 ± 1.5% |
| CVD+ | 81% | 100% | 83% | 73% | 100% | 31% | 85% | 90% |
| HF+ | 18% | 22% | 24% | 16% | 20% | 9% | 12% | 18% |
| Median F/U (years) | 3.8 | 2.1 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 | 1.8 |
| Key Findings | MACE & Renal events reduced | No impact on primary CV - Renal events reduced | 26% lower risk of MACE Renal events reduced | No impact on primary endpoint | MACE & Renal events reduced | MACE & Renal events reduced | No Significance MACE reduction | Risk of MACE & Renal events reduced |
| Y=Years; HbA1c=Hemoglobin A1c; CVD=Established Cardiovascular disease; HF=History of Heart Failure; F/U=follow-up; MACE=Major Adverse Cardiovascular Events; mg=Milligrams; µg=Micrograms | ||||||||
Table 3: List of GLP-1 RA Clinical Trials with Cardiovascular and Renal Benefits.
Based on this plethora of data, there is overwhelming support among “experts” to recommend the addition of SGLT-2i and GLP-1 RA medications to the management of T2DM patients during any and all stages of the disease. Except in some specific circumstances, i.e., allergy or intolerance to the drugs and severe reactions with serious adverse effects, these anti-diabetic agents have a safe and effective profile tested both in clinical trials and in the general population worldwide. Consistent with the principal objectives set for the management of T2DM in each period of the evolution of the disease, treatment with either SGLT-2i or GLP-1 RA agent or both early is strongly encouraged. If the initial window of opportunity is missed, a late start using either one of these medications is still advised, since most of the beneficial clinical outcomes are anticipated. It is safe to use either agent in combination with metformin or pioglitazone, as tolerated, as minimal adverse events have been reported. Although there are many clinical trials and long-term observational studies that support the efficacy and safety use of these agents in multi-drug combination regimens to treat T2DM patients, no conclusive evidence that a summation of cardiovascular and renal benefits occurs when SGLT-2i and GLP-1 RA are combined to treat T2DM.
In summary, T2DM is a chronic disease with various metabolic and hormonal disorders derived from genetic and environmental factors that compromise several organs and functional systems over time. Prevention must be initiated in the pre-diabetes phase aimed at high-risk individuals with an aggressive approach focused mainly on body weight and the excess fat. At this early stage, a concerted effort must be directed towards improving tissue insulin resistance and protecting the residual beta-cell insulin secretion. These interventions should include lifestyle modifications, healthy nutritional adjustments and pharmacotherapy, if needed, initially with either metformin and/or pioglitazone. By the time T2DM is diagnosed, the principal goal of therapy changes and the control of hyperglycemia becomes a top priority. The choice of effective anti-hyperglycemic agents becomes necessary in order to minimize acute complications, such as to avoid hospitalization for acid-base and hydro-electrolytic disturbances, as well as for acute infections or coronary events. A short trial of insulin therapy or sulfonylurea may be warranted although rapid substitution for SGLT-2i and/or GLP-1 RA agents is highly recommended. At diagnosis, a thorough evaluation of glucose-dependent microvascular complications, like retinopathy, nephropathy and neuropathies are mandatory. All measures instituted during the pre-diabetes stage should remain in place. Together with the new medications, the therapeutic goals are more likely to be achieved. As diabetes progresses, the management goals shift and focus on the reduction of the risk for development and advancement of cardiovascular and renal complications. Intensification of dietary recommendations, more specifically tailored exercise programs, physical therapy, smoking cessation and the promotion of a healthier lifestyle are crucial. All previously established therapies that have shown positive results must continue. A critical review with updates in all medications used that are known to provide cardiac and reno-protective effects must be undertaken. The ultimate management objectives in advanced T2DM are to provide quality of life and to help reduce precocious mortality with an extended healthy survival.
In conclusion, considering that type 2 diabetes is a common worldwide spread chronic disease for which there is no current “cure”, we propose an early aggressive multi-drug therapy associated with substantial lifestyle modifications and nutritional adjustments to minimize the progression and postpone the cardiovascular and renal complications of the disease. Most of these therapeutic recommendations have been extensively studied and they appear safe and efficacious. The challenge to health professionals is how to implement them properly at each stage of advancement of the disease and then, provide a close follow up to avoid harm and ensure benefits. In the decision process, it is of critical importance to involve patients and their families and, to convince third-party payers that prevention is the best prescription. The ultimate objectives in the management of type 2 diabetes are to improve the quality of life and help reduce precocious mortality with an extended healthier survival.
T2DM = Type 2 diabetes mellitus; OGTT = Oral Glucose Tolerance Test; HbA1c = Hemoglobin A1c ; IFG =Impaired Fasting Glucose; IGT =Impaired Glucose Tolerance; FH+ = Positive Family History of Diabetes; BMI = Body Mass Index; TNF-alpha = Tumor Necrosis Factor – alpha; WHO = World Health Organization; SGLT-2i = Sodium-Glucose Co-Transporter 2 Inhibitors; GLP-1 RA = Glucagon-Like Peptide 1 Receptor Analogues; DPP-4i = Dipeptidyl-Peptidase 4 Inhibitors; NHE-1 & NHE-3 = Sodium Hydrogen Exchanger 1 & 3
The authors declare no conflict of interest regarding this review manuscript.
The authors would like to express their gratitude to the nursing and administrative staff of the Texas Diabetes Institute, affiliated with the University of Texas Health San Antonio, for their contribution with the research and suggestions during the preparation of this manuscript.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Eugenio Cersosimo, Carolina Solis-Herrera and Curtis Triplitt. Current Risk Management Strategies in Type-2 Diabetes. J Diabetes Metab, 2024, 15(9): 1158.
Received: 02-Aug-2024, Manuscript No. jdm-24-33324; Editor assigned: 05-Aug-2024, Pre QC No. jdm-24-33324(PQ); Reviewed: 19-Aug-2024, QC No. jdm-24-33324; Revised: 26-Aug-2024, Manuscript No. jdm-24-33324(R); , DOI: 10.35248/2155-6156.10001158
Copyright: © 2024 Cersosimo E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.