Research Article - (2017) Volume 5, Issue 8
The systematic chemical analysis of leaves of Wrightia tinctoria were found to contain two flavonoid glycosides Kaempferol 3-O-rhamnoside and Quercetin 3-O-sophoroside and two flavonoid aglycone Kaempferol and Quercetin. The detailed UV 1H 13C NMR and Mass spectral data confirm the characterization of the above compounds. All these compounds are reported first time from the leaves of Wrightia tinctoria.
Keywords: Wrightia tinctoria, Flavonoid, Quercetin 3-0-sophoroside
Wrightia tinctoria R. Br belongs to family Apocynaceae commonly called as Sweet Indrajao, Pala Indigo Plant, Dyer’s Oleander “Jaundice Curvative tree in South India. It is distributed throughout the world and occurs abundantly in India and Burma. In deciduous forests, especially in Rajasthan, Madhya Pradesh and Peninsular India [1]. The bark of the Wrightia tinctoria is considered for antidiarrhoeal, aphrodisiac, anthelmintic, febrifuge, stomachic, toothache, tonic and dog bite [2-4]. It has got very important place in traditional healing and also is widely recognized medicinal plant [5]. Oil 777 prepared out of the fresh leaves of the plant has been assigned to analgesic, antiinflammatory and antipyretic activities and to be effective in the treatment of psoriasis [6-8]. It has anti-dandruff properties and hence used in hair oil preparations [9].
The vast number of literature found in database revealed that the extracts of different parts of Wrightia tinctoria showed significant pharmacological actions. Because there is a need for further investigations to isolate active principles that confer pharmacological action in continuation of our studies in the flavonoids of Indian medicinal plants, the leaves of Wrightia tinctoria were investigated for flavonoids and the results leading to the isolation of Kaempferol-3-Orhamnoside, Quercetin 3-O-sophoroside, Kaempferol and Quercetin.
From the alcoholic extract of the air-dried leaves, four flavonoids were isolated and characterized.
Compound (1)
Compound (1) was purple under UV changing to yellow under UV/NH3 and had λ max and Rf values characterestics of a flavanol glycoside. The presence of free 5,7 and 4’ OH groups can be established by the UV spectrum of the compound with shift reagents. The 1H NMR spectrum of the compound showed δ 6.44 [1H, dJ=1.76 Hz] and δ 6.2 [1H, dJ=1.72 Hz] which was predicted by the hydrogens at C-8 and C-6 of the A ring of the flavonoid skelton and two signals with ortho coupling at δ 6.9 [2H, dJ=8.74 Hz] and δ 7.9 [2H, dJ=8.76 Hz] for the hydrogens at C-3’, C-5’, C-2’ and C-6’of the B-ring. Presence of a sugar moiety can be characterized by the presence of an anomeric hydrogen signal at δ 5.3 [1H, dJ=2 Hz] and the appearance of an anomeric carbon signal at δ 103.5 in the 13C NMR spectrum. The methyl signal observed at δ 1.1 [3H, S] in the 1H NMR spectrum and at 18.3 in the 13C NMR spectrum indicated that the sugar moiety was rhamnose. On EIMS, it gave a peak at m/z 430 which was in good agreement with the molecular formula C21H19O10. From these data, compound (1) was characterized as Kaempferol-3-Orhamnopyranoside.
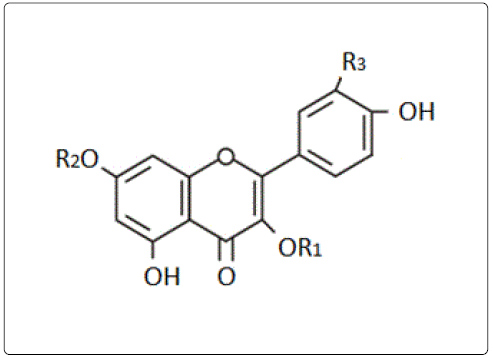
Compound (1) R1=rhamnoside, R2, R3=H
Compound (2) R1=sophoroside, R2=H and R3=OH
Compound (3) R1, R2 and R3=H
Compound (4) R1 and R2=H, R3=OH
Compound (2)
It gave yellow colour with alkalis. Pink with Mg-HCl Greenish brown with Fe3+ Positive Molisch’s test indicating it to be a flavonoid glycoside. It was purple under UV changing to yellow in UV/NH3 and had λ max (MeOH) 257, 286 sh, 358.5 nm and Rf typical of flavonol glycoside. Evidence of presence of ortho dihydroxyl group in B ring was obtained from a hyposochromic shift of 25 nm in Band I of AlCl3/HCl relative to AlCl3 spectrum. 1H NMR spectrum showed the evidence of 3,5,7,3’,4’-penta oxygenated flavone as well as the presence of protons at 2’,6’,5’,6,8 by a typical doublet pattern. The characteristic signals in the aliphatic region were assigned to the anomeric proton and other sugar protons showing the compound 2 has a diglycoside of 3,5,7,3’4’-pentaoxygenated flavone. The compound gave a molecular ion peak at m/z=626 g/mol in the EIMS. On EIMS, it gave a peak at m/ z=301 which was in good agreement with the molecular formula C15H9O7 of aglycone. These discussions led to the identification of the compound (2) was Quercetin 3-O-sophoroside.
Compound (3)
Compound (3) C15H10O6, mp 277-279°C gave pink colour with Mg- HCl, yellow with alkalis and green with Fe3+. It was yellow under UV and UV/NH3 characteristic of flavonol with free 3-OH. The presence of free 5,7 and 4’ OH groups can be established by the UV spectrum of the compound with shift reagents. A hypsochromic shift of only 8 nm in band I of AlCl3/HCl spectrum compared to AlCl3 spectrum revealed the absence of orthodihydroxyl in ring B. Further the 1H NMR spectrum showed signals at δ 12.6 for 5-OH, 10.1 for 7-OH, 7.6 (d) for H- 2’,6’, 6.92 (d) for H- 3’,5’, 6.44 (d) for H-8 and 6.19 (d) for H-6 confirms the above facts. It was also supported by 13CNMR spectrum of the compound (3). From these data compound (3) was characterized as 3,5,7,4’-tetrahydroxy flavone (Kaempferol) whose identity was further confirmed by direct comparison including co-PC with an authentic sample [10].
Compound (4)
Compound (4) had UV fluorescence and UV maxima characteristic of an aglycone flavanol. 1H NMR spectrum showed the evidence of 3,5,7,3’,4’- penta oxygenated flavone as well as the presence of protons at 2’,6’,5’,6, 8 by a typical doublet pattern. The 13C NMR spectrum of the compound further supported the above findings. Thus the structure of the flavonoid was established as a 3,5,7,3’,4’-penta hydroxy flavone or Quercetin. It was further confirmed by the direct comparison with an authentic sample from Berberis aristata [11].
Plant material
Fresh leaves (1 Kg) were collected from Lawspet, Pondicherry on July 2014 and authenticated by the Department of Botany, K.M. Centre for P.G. Studies, Pondicherry were a voucher specimen was deposited.
Extraction and isolation
The air-dried leaves of the plant were extracted thrice with boiling 95% EtOH (3Χ5L) and concentrated in vacuo to 400 mL. The aqueous extract was fractionated into Benzene, Ether, Ethyl acetate and Methyl Ethyl ketone solubles. The Benzene and ether fraction gave no characteristic spots for flavonoids and hence was not worked up further. The EAc and MEK concentrate on paper chromatography were found to contain same compounds. Hence these two were mixed and were chromatographed over a coloumn of Sephadex LH-20 using MeOH as solvent. 45 fractions of each 10 ml were collected. Compound (1) 36 mg from fractions (5-12), Compound (2) 388.9 mg from fractions (17-29), Compound (3) 86 mg from fractions (30-35) and Compound (4)126.6 mg from fractions (37-43) were obtained.
Kaempferol-3-O-rhamnopyranoside (1)
Reddish Brown coloured needles from Me2CO, mp 152-153°C (36 mg), C21H19O10, gave pink colour with Mg-HCl, olive green with Fe3+ and yellow with alkalis. It was purple under UV and changing yellow under UV/NH3. UV (λ max, nm) (MeOH): 266, 351; (+NaOAc): 274, 315.5 sh, 376.5; (+NaOAc/H3BO3) : 263.5, 366.5; (+AlCl3): 273.5, 346.5, 407.5; (+AlCl3/HCl): 267, 346.5, 392 ; (+NaOMe): 274, 317 sh , 391.5 ; 1H NMR (300 MHz, DMSO-d6, δ ppm) : 12.6 (s, 1H,OH-7), 11.2 (1H, OH-5), 7.9(d, J=8.76 Hz, 2H, H-2’,6’), 6.9 (d, J=8.74 Hz, 2H, H-3’,5’) 6.44 (d, J=1.76 Hz, 1H, H-8), 6.2 (d, J=1.72 Hz, 1H, H-6), of aglycone: 5.3 (d, J=2 Hz, 1H, H-1’’), 4.42 (dd, J=3.4, 1.5 Hz,1H, H-2’’), 3.56 (m,1H, H-3’’,4”,5”), 1.06 (d, J=6.3 Hz,3H, H-6’’) of rhamnose. 13C NMR (75.48 MHz, DMSO-d6-CDCl3, δ,ppm) 177.9 (C-4), 164.3 (C-7), 161.6 (C-5), 156.9 (C-9), 145.3 (C-4’), (C-2), 133.8 (C-3), 131.4 (C-1’), 131.3(C-3’, 5’), 115.7 (C-2’, 6’), 115.5 (C-2’), 104.4 (C-10), 101.9 (C-1”), 99.2 (C-6), 94.045 (C-8), 72.4 (C-4”), 71.6 (C-2”), 70.9 (C-3"), 68.7(C-5”), 18.3 (C-6”); MS (electrospray, relative intensity as%) 430 (M-H+,18).
Quercetin 3-O-sophoroside (2)
Yellow needles from Me2CO, (388.9 mg), C27H30O17, gave tomato red colour with Mg-HCl, olive green with Fe3+ and yellow with alkalis. It was purple under UV changing yellow under UV/NH3. UV (λ max, nm) (MeOH): 257, 286 sh, 358.5; (+NaOAc): 267.5, 378; (+NaOAc/ H3BO3) : 261, 376.5 ; (+AlCl3): 277, 433; (+AlCl3/HCl) : 277, 341, 416; (+NaOMe): 275, 323, 356 ; 1H NMR (300 MHz, DMSO-d6, δ ppm) 12.64 (s, 1H, OH-5) 10.99 (s, 1H, OH-7) 9.84 (s, 1H, OH-4’) 9.28 (s, 1H, OH-3) 8.06 (dd, J=2.2 Hz, 1H, H-2’) 7.6 (dd, J=8.3 Hz, 1H, H-6’) 6.85 (dd, J=8.4 Hz, 1H, H-5’) 6.41 (d, J=2.2Hz, 1H, H-8) 6.21 (d, J=2.2Hz, 1H, H-6), of aglycone ; 5.47 (d, J=7.6 Hz, 1H, H-1’’), 5.3 (d, J=8.8 Hz, 1H, H-2’’), 5.0 (m, 4H, H–3’’, H-4’’,H-6’’), 3.24 (m,1H1H,H-5’’), ofglucose; 5.39 (d,J=2Hz,1H,H-1’’’), 4.4 (d,J= 8.8Hz, 1H,H-2’’’), 3.45 (m,2H,H-3’’’,H-4’’’,H-6”’), 3.09 (m, 1H,H-5’’’) of glucose. 13C NMR (75.48 MHz, DMSO-d6-CDCl3, δ,ppm) 177.9 (C-4), 164.6 (C-7), 161.7 (C-5), 156.8 (C-9), 156.7 (C-2), 148.9 (C-4’), 145.3 (C-3’), 133.8 (C-3), 122.4 (C-1’), 121.6 (C-6’), 116.7 (C-5’), 116.4 (C-2’), 104.4(C-10), 102.3 (C-1”’), 101.3(C-1”) 99.1(C-6), 93.9 (C-8), 78.0 (C-2”), 76.9 (C-3”), 76.3 (C-3”’), 74.6 (C-5”), 73.6 (C-5”’), 71.6 (C-2”’), 70.3 (C-4”), 68.3 (C-4”’), 61.4 (C-6”), 60.6 (C-6”’). MS (electrospray, relative intensity as %) 625 (M-H+,10), 301 [M+- diglucose, 9].
Kaempferol (3)
Crystallized as yellow needles from Me2CO, mp. 277-279°C C15 H10O6 (86 mg). It gave pink colour with Mg-HCl, yellow with alkalis and green with Fe3+. It was yellow under UV and UV/NH3.UV (λ max., nm) MeOH : 266, 360 ; NaOAc : 271.6, 315.5 sh, 371.2 ; NaOAc/H3BO3: 262, 362; AlCl3 : 273.5, 345, 408.8 ; AlCl3/HCl: 261.7 , 343, 400 ; NaOMe : 271.8 , 325 sh, 392.8 1H NMR (200 MHz, DMSOd6, δ, ppm) 12.5 (s, 1H,OH-5), 10.1(brs, 1H, OH-7), 8.04 (d, J=8.76 Hz, 2H, H-2’,6’), 6.92(d, J=8.76 Hz, 2H, H-3’,5’), 6.44 (d, J=1.72 Hz, 1H, H-8), 6.19 (d, J=1.74 Hz, 1H, H-6). 13C NMR (50 MHz, DMSO-d6, δ, ppm) 176.00 (C-4), 164.0 (C-7), 160.81 (C-5), 159.28 (C-4’), 156.27 (C-9), 146.88 (C-2), 135.77 (C-3), 129.62 (C-2’6’), 121.78 (C-1’), 115.54 (C-3’5’), 103.14 (C-10), 98.31 (C-6), 93.59 (C-8). MS (electrospray, relative intensity as%) 285 (M-H+,89).
Quercetin (4)
Yellow needles from Me2CO, mp 305-306°C (126.6 mg), C15H10O7, gave pink colour with Mg-HCl, olive green with Fe3+ and yellow with alkalis. It was yellow under UV as well as UV/NH3. UV (λ max, nm) (MeOH): 255, 305 sh, 370; (+NaOAc): 274, 318, 386 (dec); (+NaOAc/ H3BO3) : 259, 324 sh, 380; (+AlCl3): 270, 347,437; (+AlCl3/HCl): 264, 303 sh, 356, 424; (+NaOMe): 274, 326, 400 (dec); 1H NMR (300 MHz, DMSO-d6, δ ppm) 12.5 (s, 1H, OH-5) 10.79 (s, 1H, OH-7) 10.1 (s, 1H, OH-4’) 9.6 (s, 1H, OH-3) 9.36 (s, 1H, OH-3’) 8.06 (d, J=2.2 Hz, 1H, H-2’) 7.68 (d, J=8.3 Hz, 1H, H-6’) 7.55 (d, J=8.4 Hz, 1H, H-5’) 6.92 (d, J=2.2Hz, 1H, H-8) 6.42 (d, J=2.2Hz, 1H, H-6): 13C NMR (75.48 MHz, DMSO-d6-CDCl3, δ,ppm) 176.4 (C-4), 164.3 (C-7), 161.2 (C-5), 156.5 (C-9), 148.2 (C-4’), 147.3 (C-2), 145.5 (C-3’), 136.1(C-3), 122.4 (C-1’), 120.4 (C-6’), 115.9 (C-5’), 115.5 (C-2’), 103.5 (C-10), 98.7 (C-6), 93.8 s(C-8). MS (electrospray, relative intensity as%) 301 (M-H+,100), 285 [M-OH-, 60],
The Authors are very thankful to the department of Botany, K.M. Centre for P.G. Studies, Puducherry for having authenticated the fresh leaves are collected from Lawspet Puducherry in July 2014.