Case Report - (2023) Volume 8, Issue 2
We report a rare case of a 49-year-old Omani woman who is known to have primary Anti-Phospholipid Syndrome (APS), Glucose-6-Phosphate Dehydrogenase deficiency (G6PD) and Iron Deficiency Anaemia (IDA). During cannulation she was found to develop bulla that progressed to ulcerations. With chronicity and recurrent abscess formation that usually increase after surgical intervention, a pathergy phenomenon was postulated. High suspicion of Pyoderma Gangrenosum (PG) was considered. Fortunately the rapid progression of the disease was slowed down with corticosteroid, cyclosporin and biologic agents.
Antiphospholipid syndrome; Pyoderma gangrenosum; Bullous pyoderma gangrenosum; Pathergy; Pathergic phenomenon
APS is a prothrombotic condition defined by the presence of antiphospholipid antibodies and manifests clinically as recurrent morbidity during pregnancy and/or thromboembolic complications. The cutaneous manifestations of APS vary from livedo reticularis to cutaneous necrosis [1]. Ulcers resembling PG have been described in APS and may cause confusion in diagnosis. We reported a case of a middle aged lady known to have APS and presents with pathergy among cannulation and subsequent formation of non-healing ulcers that has been relatively refractory to novel treatments. Currently our patient is being trialed on a new biologic agent that is proven to have success as described recently in the literature [2].
A 49-year-old lady is a known case of primary APS. Her past medical history includes G6PD, uterine fibroids, and chronic iron deficiency anaemia requiring frequent parenteral iron and blood transfusions.
She presented to the emergency department in February 2022 with easy fatiguability, palpitations and pallor. She was found to be very pale and an urgent complete blood count was requested. The department’s staff was informed that her haemoglobin resulted as 5 grams per deci litre (g/dl) [3].
The mean corpuscular volume was 54.9 femtoliter (fl), and the mean corpuscular haemoglobin was 18.10 pico grams per cell (pg/cell). The patient denied ingesting any fava beans or being exposed to any factors that would trigger haemolytic anemia due to her G6PD. An urgent blood transfusion was requested.
During cannulation, the cannula site developed erythema followed by bulla formation. The cannula has been changed few times to other sites with the same reaction happening in the new sites.
In the next following days the patient started to develop ulcers at the cannula sites. Later the sites have formed local abscesses. Figures 1 and 2 are early photographs of the lesions seen after cannulation. Figures 3 and4 shown early bulla formation around areas of ulceration [4].
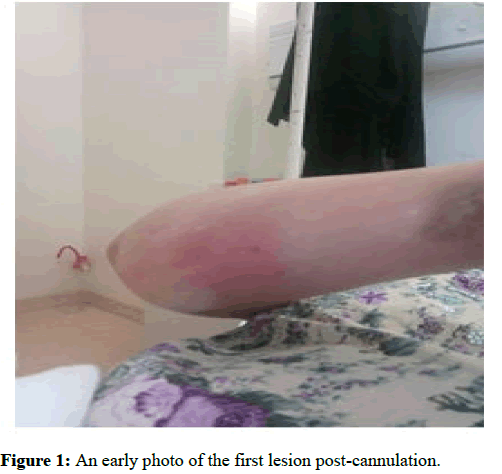
Figure 1: An early photo of the first lesion post-cannulation.
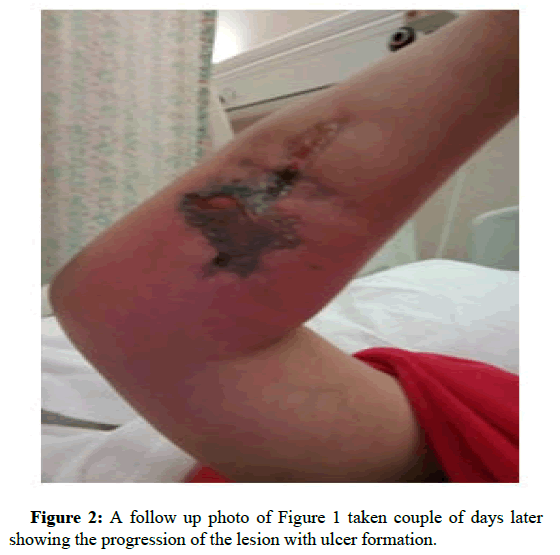
Figure 2: A follow up photo of Figure 1 taken couple of days later showing the progression of the lesion with ulcer formation. Dermatology Case Reports, Vol.8, Issue 1, 1-9 Case Report 1
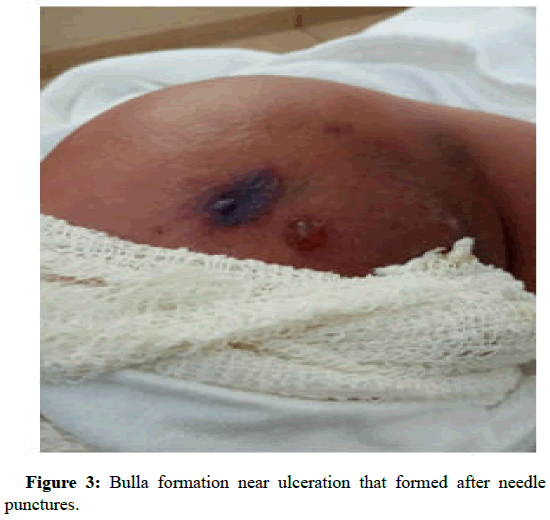
Figure 3: Bulla formation near ulceration that formed after needle punctures.
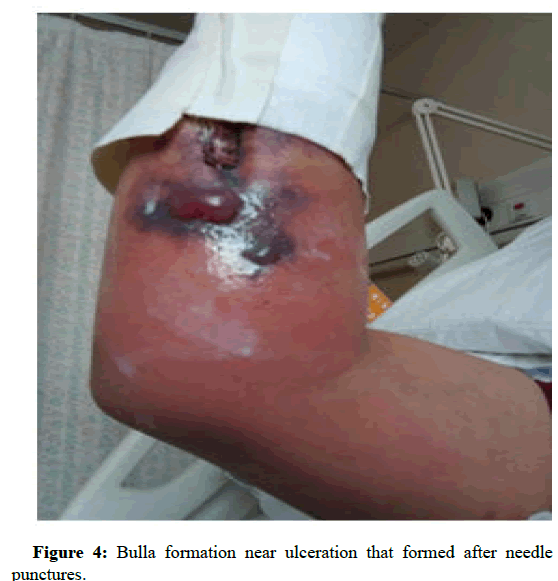
Figure 4: Bulla formation near ulceration that formed after needle punctures.
With increasing size of the abscess and failure to respond to empirical antibiotics a surgical review was sought. Soft tissue biopsy and a tissue Ultra Sonography (US) scan were requested [5]. Tissue samples sent for histological analysis were reported to be showing features consistent with acute necrotising inflammation with abscess formation extending into deep margins, and fascia suggesting possibility of necrotising fasciitis. Soft tissue ultrasound study done on the lesion reported as showing localised collections and subcutaneous oedema. All Repeated ultrasounds of the soft tissue showed the same collections. Given the above findings, a high suspicion of necrotising fasciitis was considered by the surgical team and urgent drainage and debridement was done [6].
Later it was noted that the surgical incisions had poor healing, along with progressive increase in their size. At the time multiple pus swab cultures grew pseudomonas. Few trials to insert cannulas for intravenous antibiotics ended with similar episodes of bulla followed by ulcerations. As a result the patient refused any further trials limiting her treatment only to oral antibiotics and oral iron to treat her condition [7].
Because of the above and along with the prolonged stay of the patient in the hospital that exceeded 3 months, the rheumatology and dermatology teams were requested to be involved in her care [8]. A punch biopsy was taken, and was reported as showing variable neutrophilic and mixed inflammatory infiltrate. From the history of evolvement of the lesions, along with the chronicity and the development of new lesions with any minor or major traumas (cannulation and surgical debridement’s with incisions and drainage) a pathergy phenomenon was confirmed and the patient was diagnosed with bullous pyoderma gangrenosum [9]. A decision to decrease tissue traumas and unnecessary cannulation was made. Systemic steroids were started, and later on were boosted to reach 0.7 mg/kg twice daily. Cyclosporine was added to the regimen as steroid sparing immunosuppressant with occasional intra lesional steroids for the new lesions and on sites of urgent needed cannulation. The patient was investigated thoroughly with US, CT, and no clues toward malignancy were found. A plan for bone marrow aspiration to rule out any underlying lymphoproliferative disorder was offered, but that was not feasible because of the strong pathergic reaction which led to the patient’s complete refusal of the procedure [10].
In 6 weeks time marginal response was achieved. Upon selection of biologics, infliximab was the first option but it was delayed because of the strong pathergy to canulation and patient refusal. The combined team decided to start adalimumab 40 mg subcutaneous once every two weeks. After a month of starting treatment, the patient improved, however the improvement was slow. As a result, the team decided to maximize Adalimumab to once weekly. After a month, the patient showed an estimated improvement of 70% in terms of pain, ulcers depth, erythema and general condition. The lesion healed with atrophic and cribriform scarring.
Figures 5 and 6 are a comparison between lesions seen before initiation of treatment and the same lesions after 1 month of treatment (Figure 7).
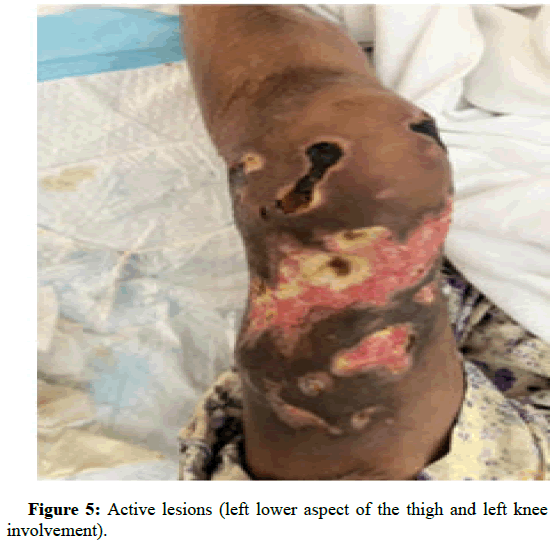
Figure 5: Active lesions (left lower aspect of the thigh and left knee involvement).
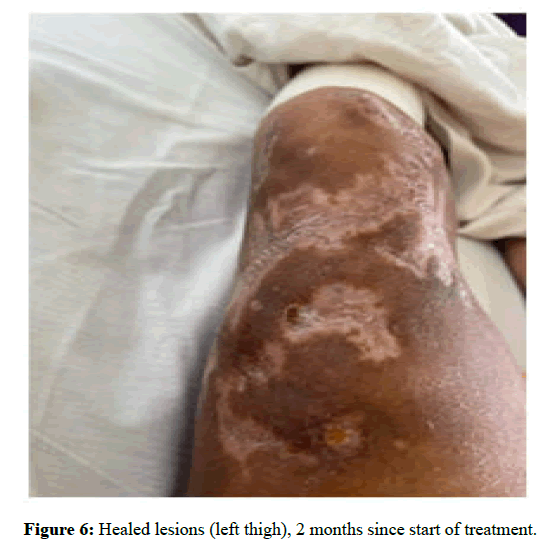
Figure 6: Healed lesions (left thigh), 2 months since start of treatment.
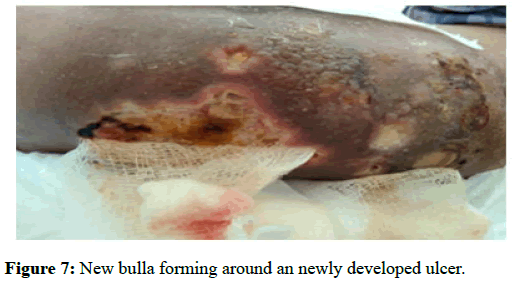
Figure 7: New bulla forming around an newly developed ulcer.
Accordingly, she was discharged on just wound care and hydrocolloid dressing (Duoderm) every three to four days. The prognosis of slow healing over months and possible recurrence were explained to her. The surgical staff and her caring teams were clearly informed of the adverse consequences of surgical incision, debridement and cannula insertion and were advised gentle superficial mild debridement for slough if required in the future.
Unfortunately, and after discharge the patient visited 2 hospitals, and she didn’t follow the instructions of the regular wound care ending up in recurrence of pathergic reactions, deep ulcerations along with superadded infections in the abdomen as well as upper and lower limbs.
All the previous wounds and surgical interventions were complicated by very low haemoglobin of 6.8 (g/dl). A general decision between all treating teams in all the hospitals was made to change the biologic agent. The patient was admitted again and same measures taken includes systemic, steroids, cyclosporin, intralesional steroids, daily dressing, frequent wound swabs, topical steroids and frequent monitoring of her vital signs and routine investigations.
While awaiting the approval of ustekinimab (the new elected biologic agent) she received anakinra 100 mg subcutaneous twice daily for a week then once daily for another week without any added benefits. With ustikinumab the patient started to show response except for the regular pseudomonal nosocomial infection which complicated the condition with frequent abscess formation. A right Femoral Venous Catheter (FVC) was placed by injecting intralesional triamcinolone and hydrocortisone in circular manner around the insertion point to avoid pathergy. IV antibiotics, IV iron and blood transfusion started. Systemic steroids were tapered slowly and she was started on a maintenance dose of colchicine 500 mg twice daily.
All abscesses were drained using small incisions and superficial debridement along with daily wound care continued. The wounds were washed with 10% acetic acid to eradicate Pseudomonas.
Pyoderma Gangrenosum (PG) is a rare dermatological inflammatory condition triggered by dysfunctional neutrophils, thus, classified as a neutrophilic dermatosis. Histological predominance of neutrophils in the affected lesions and absence of any bacterial source for the inflammation are indicative features. A PG lesion typically starts as a pustule or a blister, often triggered by skin injury (the pathergic response). The lesion then progresses to form painful ulcers with undermined, violet coloured borders. Subtypes of PG include bullous, ulcerative, pustular and vegetative. The bullous form is characterized by rapidly spreading painful superficial bulla that breakdown to form ulcers. It commonly involves the upper extremities and trunk, and is linked to myeloproliferative disorders. Ulcerative PG is usually seen on the lower extremity at the site of skin trauma and starts as non-erythematous pustules that progresses to form an ulcers. The pustular form initially starts as painful erythematous pustule with an erythematous base. Finally, the vegetative type starts with an ulcer that could form sinus tracts. It is reported to have good response to treatment.
PG is diagnosed clinically by its appearance and the severe pain associated with it. The Maverakis criteria comprises a single compulsory major criterion and eight minor criterions as shown below Table 1. With the presence of more than four minor criterions it yields good sensitivity and specificity.
| Diagnostric criteria for pyoderma gangrenosum | |
|---|---|
|
|
Histology of the ulcer show neutrophilic infiltrate. |
|
|
Infectious causes ruled out. |
| Pathergic phenomenon confirmed. | |
| History of systematic disease including Inflammatory bowel disease or inflammatory arthritis present. | |
| Evidence of papule, pustule or vesicle ulcerating within 4 days since appearing. | |
| Peripheral oedema, undermining borders and tenderness at ulceration site. | |
| Multiple ulcerations, at least one on an anterior lower extremity. | |
| Cribriform or wrinkled paper scar(s) at healed ulcer sites. | |
| Reduction in ulcer size within one month of initiation of immunosuppressive therapy. | |
Table 1: Diagnostic criteria for pyoderma gangrenosum.
Evidence accumulated over the years display a strong link between PG and systematic diseases. In fact it is reported that more than 50% of cases of PG have underlying systematic disorders. This association with systematic diseases supports the auto-inflammatory speculation and the role of the innate immunity in the pathogenesis of PG. Idiopathic PG has also demonstrated auto inflammatory features by good response of cases to systemic immunosuppressive therapy. Review of recent literature clearly demonstrates a relationship between PG and several disorders including IBD, arthritis and haematological malignancies, and a rare association with systemic lupus erythmatosus was reported. However, there are only few cases of PG that have been described in Anti- Phospholipid Syndrome (APLS).
Among the most recent cases reported in the literature is a 39 year old male who presented with necrotic erosive lesions over the legs. Lab investigations revealed high anti-cardiolipin, anti-beta glycoprotein and detectable lupus anticoagulant. The authors suggest that the pathogenesis of PG-like cutaneous ulcer are related to some abnormalities in the coagulation/fibrinolysis pathways seen in APLS. Another recent case of a 35-year-old female presenting with a non-healing ulcer for 3 months and was incidentally found to have APLS with no previous disease manifestations. Those cases suggest that clinicians should consider APLS in first time presentation with PG-like skin lesions.
APLS is an autoimmune disorder characterized by tendency to develop thrombi and recurrent abortions, and has long been related to various cutaneous manifestations including livedo reticularis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis and cutaneous ulceration. Although PG has never been described as a cutaneous manifestation of APLS, the features of the ulcerations seen are described to be deep, painful and located above the malleoli. Researchers investigated lower extremity ulcerations of a group of APLS confirmed patients and found features of PG in 15% of the cases. Those were treated successfully with immunosuppressive therapy (azthioprine or cylcosporin). This might suggest that PG could be overlooked in APLS and is a call for more research that focuses on PG in APLS.
General measures that must be taken when managing PG include local wound care with frequent cleansing and dressing that preferably provides moist non adherent environment for the lesions. In PG it is also essential to avoid pathergic phenomena either by trauma or any substances applied to the skin. Surgical intervention poses a risk for pathergy, so it should only be limited to superficial gentle debridement when necessary.
The first line management in limited PG can be started with topical or intralesional corticosteroids, or even topical calcinurine inhibitors. The treatment can be systemic corticosteroids if the PG disease has more extensive lesions. Systemic cyclosporine is used as a substitute first line if steroid are not tolerated or is added as an adjunct therapy with steroids.
Biologic and conventional immunosuppressive agents, and antibiotics are considered as second line therapy in PG and it used patients that have not responded to first line management. A variety of biologic agents such as infliximab, adalimumab, etanercept, certolizumab and golimumab has been used and patient exhibit improvement dramatically when combined with other systemic therapy. Conventional immunosuppressive agents such as mycophenolate mofetil, methotrexate, and azathioprine have been used in treatment of PG. in addition, antibiotics, such as dapsone and minocycline, also showed a role in treatment of some case of PG. Dapsone has been avoided in our case as it increased risk of drug induced hemolytic anemia when used in G6PD cases.
Moreover, growing research and trials have yielded successful management results with new agents such as ustekinumab, canakinumab, and anakinra. The success in management using ustekinumab was demonstrated in six refractory Pyoderma gangrenosum patients followed up in an Australian health institution located in Monash, Melbourne. The authors report an effective and safe treatment profile with no adverse reactions recorded.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Albahrani M, Alweshahi Y, Almoqbali A, Alajmi. "Bullous Pyoderma Gangrenosum Rare Association: A Case Report ". Dermatol Case Rep, 2023, 8(1), 1-4.
Received: 05-Feb-2023, Manuscript No. DMCR-23-21712; Editor assigned: 07-Feb-2023, Pre QC No. DMCR-23-21712 (PQ); Reviewed: 21-Feb-2023, QC No. DMCR-23-21712; Revised: 21-Apr-2023, Manuscript No. DMCR-23-21712 (R); Published: 28-Apr-2023, DOI: 10.37532/2684-124X.23.8.1.006
Copyright: © 2023 Albahrani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.