Research Article - (2018) Volume 6, Issue 3
Received Date: Feb 06, 2018 / Accepted Date: Feb 18, 2018 / Published Date: Mar 01, 2019
Freeze-dried rhubarb was fractionated using column chromatography with organic solvents (various ratios of water/methanol and acetone). The seven fractions obtained were examined for antioxidant activity using a gas chromatography/malonaldehyde (GC/MA) assay. Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) in each fraction was analyzed by HPLC with a diode array detector. The amounts of emodin found in the seven fractions ranged from 22.4 ± 0.35 μg/g of dried rhubarb (100% methanol eluate) to 0.33 ± 0.01 μg/g (acetone eluate). Emodin was not detected in the fractions eluted with water/methanol mixtures. Among the seven fractions, the fraction eluted with 100% methanol showed the greatest antioxidant activity and contained the highest level of emodin. This fraction exhibited antioxidant activities by 75.4 ± 13.7% at the level of 50 μg/mL, 92.8 ± 0.6% at the level of 100 μg/mL, and 93.9 ± 1.1% at the level of 500 μg/mL. These levels of antioxidant activity were comparable to those of known antioxidants α-tocopherol and BHT. The strong relationship between amounts of emodin in the fractions and antioxidant activity was observed.
Keywords: Emodinn HPLC, Natural antioxidants, Polyphenols, Rhubar
The rhubarb plant (Rheum rhabarbarum L.), which belongs to the Polygonaceae family, grows wild in Asia and is cultivated in greenhouses in many countries in the world. Rhubarb is widely used as an ingredient of food products, including pies, cakes, muffins, breads, jams and salad. In addition, rhubarb has been used as a traditional medicine in China and Japan for many years. It is an official prescription drug in Europe [1-3]. Rhubarb reportedly has pharmacological activities such as antitumoral, antimutagenic, antiinflammatory, and anticarcinogenic [4,5]. Many chemicals have been identified in rhubarb, including anthraquinones, dianthrones, naphthalins, stilbenes, galloyglucoses, anthocyanins, flavonoids, polyphenols, organic acids and acylglucose derivatives [6,7]. Among them, anthraquinones such as emodin, aloe-emodin, rhein, and physcion have been reported as components having bioactivities, such as antioxidant [1,8,9].
Recently, studies searching natural plants with antioxidant activity have become considerably more popular because the use of synthetic antioxidants, such as butylated hydroxyl toluene (BHT), have been restricted due to their toxicity [10,11]. The antioxidant scavenges reactive oxygen radicals, such as a hydroxyl radical, which cause oxidative damage to cell membranes and subsequently lead to various diseased including cancer, Alzheimer's disease, arthritis, diabetes, atherosclerosis, and AIDS [12]. Therefore, discovery of compounds having antioxidant activity in natural plants is in order.
Less volatile components of rhubarb plants, such as emodin, have been analyzed by high performance liquid chromatography with a diode array detector (HPLC/DAD) [7,13,14], liquid chromatography with mass spectrometric detector (LC/MS) [7,13,14], thin-layer chromatography (TLC) [15] and capillary electromigration (CE) [16]. In the present study, organic solvent extracts from rhubarb and its main component, emodin, were analyzed by HPLC/DAD and their antioxidant activity was evaluated by a gas chromatography/ malonaldehyde (GC/MA) assay [17].
Chemicals and materials
α-Tocopherol (vitamin E), N-methylhydrazine (NMH), 1-methyl pyrazole (1-MP), 2-methylpyrazine (2-MP), sodium dodecyl sulfate (SDS), and ferrous chloride were purchased from Sigma Chemical Co. (Milwaukee, WI). Cod liver oil (approximately 70% ω-3 fatty acid methyl esters), butylated hydroxytoluene (BHT), trizma hydrochloride, and trizma base were bought from Sigma Chemical Co. (St. Louis, MO). Hydrogen peroxide, hexane, chloroform, ethyl acetate, methanol, acetone, and purified water were purchased from Fisher Scientific Co., Ltd. (Fair Lawn, NJ). A standard solution of 2-MP was prepared by adding 100 mg of 2-methylpyrazine to 10 mL of ethyl acetate and was stored at 4°C.
Rhubarb was bought from a local market in Davis, California and its stalks (approximately 1 kg) were freeze dried at -48°C and 0.05 m Bar for 48 h (FreezeOne, Labconco, MA, USA). The dried rhubarb was ground in a blender to give a fine white powder (45 g).
Fractionation of rhubarb by column chromatography for chemical analysis and antioxidant evaluation
The dried rhubarb powder (15 g) was placed in a glass column (40 cm × 4.5 cm i.d.) packed with Amberlite XAD-2 resin (Supelco, Bellefonte, PA). The column was eluted sequentially with 500 mL each solvent-water/methanol=100/0 (Fraction I), 80/20 (Fraction II), 60/40 (Fraction III), 40/60 (Fraction IV), 20/80 (Fraction V), and 0/100 (Fraction VI), and subsequently with 100% acetone (Fraction VII). The solvent of each fraction was removed using a rotary flash evaporator under reduced pressure and then freeze-dried at -48°C for 24 h. Freeze-dried samples were stored at -5°C for further experiments.
Isolation and purification of emodin (1,3,8-trihydroxy-6- methylanthracene-9,10-dione) from dried rhubarb
Isolation of emodin from rhubarb was conducted by a slightly different method from the one used for fractionation. The fractionation method used did not provide sufficient amounts of emodin for further experiments.
The dried rhubarb powder (5 g) was dissolved in 250 mL of deionized water. The aqueous solution was sequentially extracted with 250 mL each of hexane, chloroform, and ethyl acetate using a 1 L separatory funnel. The solvent of the extracts was removed using a rotary flash evaporator to approximately 10 mL under reduce pressure. Each extract was freeze-dried at -48°C for 24 h. The amount of condensed solvent extracts was 558 mg from hexane, 169 mg from chloroform, 92.0 mg and 89.2 mg from ethyl acetate.
The chloroform extract was placed in a 15 cm × 1 cm i.d. glass column packed with 5 g of 100-200 mesh silica gel (Avantor performance materials, Inc., Philipsburg, NJ). Anhydrous sodium sulfate (1 mg) was placed on top of the silica gel to remove trace water. The column was washed with 30 mL hexane/ethyl acetate (4/1, v/v) and then eluted with 100% methanol. Methanol was removed by a rotary evaporator under reduced pressure and then purified nitrogen stream to yield 51.0 mg of purified emodin, which was stored at 5°C for further experiments.
Gas chromatography/malonaldehyde (GC/MA) antioxidant assay on fractions from rhubarb
The antioxidant activities of fractions and purified emodin were determined by analyzing MA formed from cod liver oil upon oxidation after derivatizing to 1-methylpyrazole (1-MP) with N-methylhydrazine (NMH) according to a previously reported method [17]. An aqueous solution (5 mL) containing 30 μL cod liver oil, 0.25 mmol trizma buffer (pH 7.4), 1 μmol ferrous chloride, 2 μmol hydrogen peroxide, 0.75 mmol potassium chloride and 1% surfactant SDS was incubated with various amounts of rhubarb extracts and emodin for 17 h at 37°C in a 20 mL test tube. The oxidation of the samples was stopped by the addition of 50 μL 4% BHT solution. NMH (30 μL) was added to the oxidized cod liver oil solutions, and the solutions were stirred for 1 h at room temperature. 1-MP formed in the solutions was extracted using a solid phase extraction (SPE) cartridge (MEGA BE-C18, 1 g, 6 mL, Varian, USA). 2-Methylpyrazine (20 μL) was added to the extract as a GC internal standard and the volume of the extract was adjusted to exactly 10 mL with ethyl acetate. The solution was analyzed for 1-MP using a gas chromatograph with a nitrogen-phosphorus detector (NPD). Standard antioxidants, BHT and α-tocopherol were used to verify the assay used. A blank sample was prepared without test material. Antioxidant activity (%) was calculated by the following equation:
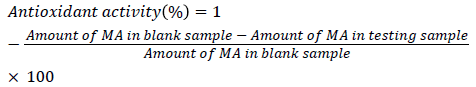
Analysis of emodin in fractions from rhubarb
The amount of emodin in the seven fractions obtained from freezedried rhubarb was quantified by HPLC/DAD. The linearity value (R2) of the standard curve for quantitative analysis was 0.9999 for emodin. Total analysis time was 65 min. Figure 1 shows a typical HPLC of Fraction VI, which had the strongest antioxidant activity (discussed below). The retention time of emodin was 36.1 min. Identification of the emodin peak was confirmed by matching retention time and mass spectral fragmentation patterns [7,14]. Mass spectra of emodin was compared with the literature for the identification in the ESI negative ion mode (m/z=269).
Determination of total phenolic content
The total phenolic content was determined according to Folin- Ciacalteu’s method [18]. Briefly, 0.1 mL of each fraction (500 μg/mL) was added to a 2% sodium carbonate solution (2 mL) and allowed to stand for 3 min. After addition of a 50% Folin-Ciacalteu’s solution (0.1 mL), it was allow to stand for 30 min at room temperature. After the reaction, the absorption activity was measured at 760 nm using a UVvisible spectrophotometer. All experiments were carried out in triplicate. Total phenolic content was calculated using a calibration curve with gallic acid. The result was demonstrated with mg gallic acid equivalent/g extract.
Instrumental analysis
An Agilent model 1100 HPLC series (Agilent Technology, Palo Alto, CA) equipped with a reversed phase column (Capcell pak C18, 75 mm × 4.6 mm, 3 μm, Phenomenex, Torrance, CA) and a diode array detector (set at 280 nm) was used for emodin anlysis. The injection volume was 10 μL and flow rate was 0.4 mL/min. The mobile phase consisted of 0.1% acetic acid in water and methanol (solvent A) and acetonitrile (solvent B). The linear solvent gradient was from 5% B to 100% B in 65 min. Emodin was identified using an Agilent model 1100
HPLC interfaced to an Applied Biosystems API 2000 MS/MS via an electrospray ionization (ESI) source operating in the negative ion mode at 400°C with nitrogen gas. The MS was operated in full scan mode within the range of m/z 50 to m/z 1000 and the extracted ion (m/z 269) was used for confirmation of emodin.
A standard curve for emodin quantitation was prepared using different concentrations of standard emodin (0.5, 1, 5, 10, 25, 100 μg/mL) in HPLC grade acetonitrile, and each solution (10 μL) was injected into HPLC. Quantification of emodin was performed with an external standard calibration curve using peak area with non-weighted linear regression.
An Agilent Model 6890 GC equipped with a 30 m × 0.32 mm i.d. (df=0.25 μm) DB-WAX bonded-phase fused silica capillary column (J and W Scientific) and an NPD were used for the analysis of 1-MP for the GC/MA antioxidant assay. The injector and detector temperatures were 250°C. The oven temperature was programmed from 60°C to 160°C at 4°C/min and then held for 2 min. The linear velocity of the helium carrier gas was 28 cm/sec at a split ratio 20:1.
Antioxidant activities of seven fractions from rhubarb
Figure 2 shows the antioxidant activity of the seven fractions obtained from rhubarb. Values are mean ± SD (n=3). All samples were tested at levels of 50 μg/mL. The result of standard antioxidants BHT and α-tochopherol were 91.0 ± 0.67% and 83.35 ± 1.83%, respectively, indicating that the method was valid. Among the rhubarb samples, Fraction VI, which contained the greatest level of emodin, showed the highest antioxidant activity (75.4 ± 13.7%) followed by Fraction II (65.7 ± 1.2%), Fraction V (63.4 ± 2.5%) and Fraction I (56.2 ± 9.5%).shows the antioxidant activity of the seven fractions obtained from rhubarb. Values are mean ± SD (n=3). All samples were tested at levels of 50 μg/mL. The result of standard antioxidants BHT and α-tochopherol were 91.0 ± 0.67% and 83.35 ± 1.83%, respectively, indicating that the method was valid. Among the rhubarb samples, Fraction VI, which contained the greatest level of emodin, showed the highest antioxidant activity (75.4 ± 13.7%) followed by Fraction II (65.7 ± 1.2%), Fraction V (63.4 ± 2.5%) and Fraction I (56.2 ± 9.5%).
Figure 3 shows the results of the antioxidant test on Fraction VI. The values are mean ± SD (n=3). Because Fraction VI showed the strongest antioxidant activity among the fractions, its activity was tested further at three different levels. Standard antioxidants BHT (92.2 ± 0.4% at 100 μg/mL and 95.3 ± 0.2% at 500 μg/mL) and α-tocopherol (84.8 ± 1.5% at 100 μg/mL and 89.9 ± 1.4% at 500 μg/mL) exhibited potent antioxidant activities, indicating that the assay used was valid. Fraction VI showed strong, clearly dose-dependent antioxidant activity (72.6 ± 6.7% at 20 μg/mL, 92.8 ± 0.6% at 100 μg/mL and 93.9 ± 1.1 at 500 μg/mL %). The strong antioxidant activity of fraction VI may be related significantly to the presence of emodin. A previous study reported that methanol extract of rhubarb exhibited antioxidant activity by 83.93 ± 2.35% at a level of 100 μL/mL [19]. These results strongly indicate that rhubarb contains potent antioxidants.
Antioxidant activity of purified emodin and its analysis in fractions from rhubarb
Figure 4 shows the antioxidant activity of emodin along with standard antioxidants α-tocopherol and BHT. The values are mean ± SD (n=3). Standard antioxidants BHT and α-tocopherol exhibited antioxidant activity of 89.18 ± 3.83% and 80.52 ± 3.51%, respectively, at the level of 20 μg/mL, indicating that the assay was valid. Emodin exhibited dose-dependent antioxidant activity. Emodin exhibited antioxidant activity of 92.1 ± 1.0% at a level of 200 μg/mL, which is a comparable activity to that of BHT at a level of 20 μg/mL. Even at a level of 20 mg/mL, it exhibited moderate antioxidant activity (32.5 ± 15.7%). These results are consistent with a previous study [1], which reported that emodin in rhubarb exhibited significant antioxidative activity toward oxidation of low-density lipoprotein.
Fraction VI had the greatest amount of emodin (22.4 ± 0.35 μg/g of dried rhubarb), followed by fraction V (1.73 ± 0.03 μg/g) and fraction VII (0.33 ± 0.01 μg/g). Emodin was not found in fractions I-IV.
Total phenolic contents of seven fractions from rhubarb
Figure 5 shows the total phenolic contents of the seven fractions extracted from rhubarb. The values are mean ± SD (n=3) (mg gallic acid equivalent/g of extract). Fraction IV exhibited the highest level (88 ± 1.5 mg/g), followed by Fractions I (71 ± 1.6 mg/g), II (67 ± 1.6 mg/g), III (74 ± 1.2 mg/g), V (73 ± 1.5 mg/g), and VII (57 ± 0.1 mg/g). Fraction VI exhibited the lowest level of total phenolic content (42 ± 0.8 mg/g). The significant correlation coefficient between total phenolic content and antioxidant activity was reported previously [20]. Clear correlation between phenolic content and antioxidant activity was not observed. Fraction VI, containing the highest level of emodin, showed the highest antioxidant activity in the present study. These results suggest that the amount of emodin rather than the total phenolic content plays an important role in the antioxidant activity of rhubarb.
The results of the present study demonstrated that rhubarb extracts exhibit potent antioxidant activity, which is comparable to those of standard antioxidants, α-tocopherol and BHT. A strong relationship between emodin and antioxidant activity was also observed. Therefore, the results of the present study suggest that rhubarb is a valuable source of natural antioxidants. However, previous and the present studies suggest that there are many other antioxidant compounds in rhubarb. Further work on identification of antioxidants in rhubarb, in addition to emodin, is in order.