Research Article - (2023) Volume 11, Issue 1
In Mali, Acacia nilotica is widely used in traditional medicine in the management of many diseases including liver diseases. The objective was to estimate the toxicity and to evaluate the analgesic and anti-inflammatory effects of aqueous (infused) extracts of leafy twigs, stem barks and fruits of A. nilotica. Toxicity was estimated according to OECD guidelines. The analgesic activity of the extracts was evaluated on the pain caused in mice or rats by acetic acid, formalin and hot water. The anti-inflammatory effect was determined on carrageenan edema in the paws of rats. Orally infused at 5000 mg/kg didn’t cause mortality in rats. Infused from all 3 organs demonstrated a significant reduction in pain and inflammation caused. The stem bark infused gave the best analgesic and anti-inflammatory activity in different tests performed. For the stem bark extract, the number of writhing was 24.80 + 4.27, comparable to that of paracetamol at 100 mg/kg. In the late phase of the formalin test, the same extract gave a percentage inhibition of 68.14% higher than 66.56% for acetylsalicylic acid at 100 mg/kg. For heat-induced pain, there was a 20.7% increase in tail withdrawal time from hot water compared to the negative control batch. The stem bark infused dose of 400 mg/kg exhibited inflammation inhibition percentages of 58.57%; 61.41% and 67.28% respectively at 1 hour, 3 hours and 5 hours after injection of carrageenan. These analgesic and anti-inflammatory properties of aqueous extracts of A. nilotica, in particular the stem bark, can contribute to the management of painful and inflammatory symptoms associated with liver disease.
Mali • Acacia nilotica • Analgesic and anti-inflammatory activity • Liver disease
Liver diseases are diseases characterized by damage to the liver, thus affecting its normal functioning. They are becoming more and more frequent throughout the world and more particularly in Africa. There is a wide range of liver diseases which are among others, hepatitis, cirrhosis, cholestasis, steatosis, tumors, abscesses. Most of these diseases are characterized by inflammation and/or pain [1–3]. Hepatitis, which is the most common form of liver diseases, is inflammation of the liver that can have several causes, including viruses [4,5]. Liver diseases constitute a major health problem because of their morbidity and mortality. Most liver diseases progress to chronicity and give rise to cirrhosis when not treated normally. Cirrhosis was the twelfth leading cause of death worldwide in 2013 [6]. Viral hepatitis caused 1.34 million deaths in 2015. Hepatitis B is highly endemic in West Africa, with a prevalence of 8 %, the highest in the world [7].
The management of liver diseases is nowadays based on the use of some molecules such as interferons and antiretroviral which have excessively high costs and are therefore not within the reach of the average population. These drugs have also shown some limitations in the treatment of viral hepatitis, particularly hepatitis B. In this context, the use of medicinal plants is an important alternative path to explore in order to validate traditional uses. In Mali, a large number of people with liver disease use the resources of traditional medicine. A survey carried out at the hepato-gastroenterology department of the Gabriel Touré hospital shows that 62.3 % of patients with liver disease use medicinal plants, particularly in the management of cirrhosis [8]. For consultations in one year, at the Medical Sciences service of the Traditional Medicine Department, viral hepatitis represented 24.79% of pathologies (150/605) with 135 cases of viral hepatitis B. Among the main plants used at DMT and elsewhere, there is Accacia nilotica (Mimosaceae) sought in traditional medicine in the treatment of many conditions including liver disease. The fruits, in infusion, are used in the treatment of hepatitis in Senegal [9]. The seeds, in infusion, treat jaundice and in decoction, are used for the management of hepatitis in Togo and liver ailments in Nigeria [10,11].
Previous studies conducted on plant samples have demonstrated numerous properties including hepato-protective, anti-inflammatory and analgesic, anticancer, antioxidants and antiviral against hepatitis C virus C [12-22].
The present study aimed to determine the analgesic and anti-inflammatory properties of samples of leaves, stem, bark, and fruits of Accacia nilotica harvested in Mali.
Plant material
It was made up of samples of leafy twigs, stem bark and fruits of Acacia nilotica harvested in November 2020 in Kati. The samples were identified by Mr. Seydou M Dembélé, water and forest engineer, head of the ethnobotanical and raw materials department of the Traditional Medicine Department (DMT) in Bamako. A specimen of the plant is available from the DMT Herbarium under number 3077/DMT. The collected samples were then dried in the shade for 3 weeks before being pulverized and kept for extraction.
Animal material
The animals used were Wistar rats weighing between 155 - 210 g and Albino Swiss male and female mice weighing between 24 - 32 g. The animals were kept under standard laboratory conditions (25°C and a light/dark cycle, i.e. 12/12) and fed standard prepared at DMT and tap water. Animal welfare requirements were strictly considered during these experiments.
Preparation of aqueous extracts
For the preparation of the infused, 100 mL of boiling water were poured into an Erlenmeyer flask containing 100 g of powder of each sample. The infusion time was 15 minutes. The infused obtained after filtration on cotton, were frozen then freeze-dried.
Acute toxicity
It was assessed using the sequential method reported by the Organization for Economic Cooperation and Development [23]. Female rats were weighed and randomly divided into groups of three. They were then deprived of food but with free access to water for 14 hours. The infusions of leafy twigs, stem barks and fruits were administered by gavage sequentially at doses of 5, 50, 300, 2000 mg/kg and 500 mg/kg. After administration, the rats were again deprived of food for 3-4 hours. The rats were observed for the first four hours after administration and daily for 14 days. The observations related to the modifications of the skin, the hairs, the eyes, the mucous membranes, and the respiratory system, the activity of the autonomic and central nervous system, the somato-motor activity, behavior. There was also place to note tremors, convulsions, onset of diarrhea, lethargy, sleep.
Study of analgesic activity Writhing test
The method used was that reported by Siegmund et al. (1957) [24]. The mice were randomly divided into 11 groups of 5 mice and fasted 16 hours before the start of the test. The infusions of leafy twigs, stem barks and fruits of A. nilotica were each administered at single doses of 400, 200 and 100 mg/kg. Distilled water (10 mL/kg) was used as a negative control and paracetamol (100 mg/kg) as a reference analgesic drug. One hour after administration of the treatments, the animals received intraperitoneally 10 mL/kg of a 0.6% solution of acetic acid in physiological saline. Five minutes (5 min) after the injection of acetic acid, the number of writhings was counted in each mouse for twenty minutes (20 min).
The analgesic effect was evaluated according to the following formula:
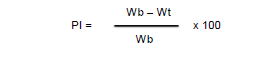
PI: percentage of pain inhibition
Wb:mean number of writhings of mice from the negative control batch
Wt: mean of the number of writhings of the mice of the batches treated with the extracts and the paracetamol.
Formalin test
Male and female rats were randomly divided into groups of 5 and fasted on food (with free access to water) for 16 hours before the start of the test.
The batches having received the infused and the negative control batch were treated as in the previous tests. Acetylsalicylic acid (100 mg/kg per os) and morphine (10 mg/kg intraperitoneally) were used as reference products. One hour after administration of the treatments (30 minutes for morphine), 20 μl of 5 % formalin were injected into the sole of the right hind paw of the rats. Then the reaction of the animals to pain (licking and biting of the injected paw) was counted for 30 min in two phases: 0 to 5 minutes (early phase), 15 to 30 minutes (late phase). The percentage of pain inhibition was calculated was calculated for each phase with the following formula [25]:
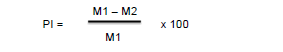
Hot water tail immersion test: This test was carried out according to the method used by Ramabadran et al in 1989 and modified by Gawel et al in 2018 [26,27]. The rats were fasted before the start of the test as previously. Before the administration (T = 0) of the extracts, 3 centimeters of the tail of each rat was immersed in water maintained at 55oC and the retraction time was noted. The infused, distilled water and morphine were then administered equally as in the previous test. The time between immersion and tail withdrawal was recorded as 30; 60; and 90 minutes after treatment administration. To avoid tissue damage in the tail, the limit time was set at 20 seconds. The pain inhibition percentages were calculated by applying the following formula:
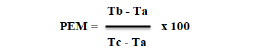
PEM: percent maximum possible effect
Tb: mean withdrawal time after administration of extracts or morphine
Ta: mean withdrawal time before administration of extracts or morphine
Tc: limit time (20 s)
Anti-inflammatory activity
The extracts were administered under the same conditions and at the same doses as before. The reference drug used was acetylsalicylic acid at 100 mg/kg. One hour after administration of the treatments, edema (inflammation) was induced by administration of 50 µL of a 1 % solution of carrageenan in physiological saline under the plantar aponeurosis of the right hind paw of rats. Then the volume of the right hind paw of each rat was measured 1 hour, 3 hours and 5 hours after the injection of carrageenan [28]. The anti-inflammatory activity was evaluated as the percentage reduction in edema in treated rats compared to white controls according to the following formula:

PI: percentage inhibition of inflammation
A: mean increase in paw volume of rats from the negative control group
B: mean increase in paw volume of the rats of the treated groups.
The data obtained were analyzed with GraphPad socket 5 software. The results were expressed as means + SEM (Standard Error of the Mean). Comparisons were performed by ANOVA One way followed by multiple comparison tests. The difference was considered significant if p < 0.05.
The research protocol of the thesis obtained the favorable opinion of the ethic committee of the Faculties of Medicine and Odonto-Stomatology (FMOS) and pharmacy (FAPH) at its session of November 11, 2021 (N° 2021/273 /CE/USTTB).
Security data
Orally, infused at 5000 mg/kg didn’t cause any noticeable change in behavior in rats or death.
Analgesic activity
According to the writhing test
The effect of the infused on abdominal torsion induced by acetic acid is shown in Table 1. Figure 1 represents the percentage of protection of the extracts against pain. The infused significantly reduced the number of acetic acid-induced writhings in mice. The best inhibitory activity (71.43%) was obtained with the stem bark infused at a dose of 400 mg/kg per os (Table 1 and Figure 1).
Table 1. Effect of extracts on the number of writhings caused by acetic acid in mice.
| Extract | Dose (mg/kg) | Number of writhings |
|---|---|---|
| Negative control | - | 86.6 + 16.12 |
| Paracetamol | 100 | 24 + 4.74*** |
| Twigs leafy | 400 | 65.20 + 7.46* |
| 200 | 73.20 + 10.62 | |
| 100 | 67.60 + 8.88 | |
| Stem bark | 400 | 24.80 +4.27 *** |
| 200 | 57.60 + 9.18** | |
| 100 | 53.40 + 7.37*** | |
| Fruit | 400 | 62.60 + 5.37* |
| 200 | 75.20 +17.25 | |
| 100 | 79.00 + 7.48 |
*** P < 0.001 treatments vs negative control ; ** P < 0.01 treatments vs negative control; * p < 0.05 treatments vs negative control
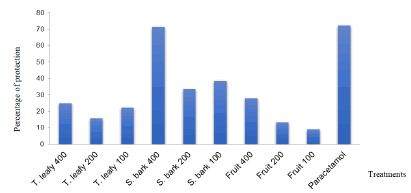
Figure 1: Percentage of protection of extracts against pain induced by acetic acid in mice. T : twigs , S : stem
According to formalin test
The results of the formalin test show that only the stem bark infused at 200 mg/kg exhibits significant activity (p < 0.05) compared to the negative control group with a percentage reduction in pain of 21.88 % during the early phase. Twigs leafy, stem barks and fruits infused significantly reduced formalin-induced pain during the late phase. The best activity was obtained with the stem bark infused at a dose of 400 mg/kg per os with a percentage inhibition of 68.14% (Table 2 and Figure 2). Morphine was 100 % effective during both phases while aspirin acted significantly only in the late phase (66.56%).
Table 2. Effect of extracts of A. nilotica on the time (in seconds) of licking and biting the paws of rats injected with formalin.
| Extract | Dose (mg/kg) | Early phase | Late phase |
|---|---|---|---|
| Negative control | - | 65.8 + 7.5 | 190.2 + 11.5 |
| Morphine | 10 | 0*** | 0*** |
| Aspirin | 100 | 56.4 + 5.59 | 63.6 + 12.9*** |
| Twigs leafy | 400 | 64.8 + 7.01 | 124.2 + 12.52*** |
| 200 | 57 + 7.04 | 163.4 + 11.48*** | |
| 100 | 58.8 + 1.64 | 160.4 + 12.58*** | |
| Stem bark | 400 | 53.4 + 7.89 | 60.6 + 8.11*** |
| 200 | 51.4 + 5.9* | 92.2 + 9.63*** | |
| 100 | 65.4 + 10 | 154 + 16.9*** | |
| Fruit | 400 | 65 + 8 | 143 + 11.77*** |
| 200 | 65.25 + 6.18 | 170.2 + 11.9* | |
| 100 | 61 + 6.83 | 180 + 15.6 |
*** P < 0.001 treatments vs negative control; ** P < 0.01 treatments vs negative control; * p < 0.05 treatments vs negative control
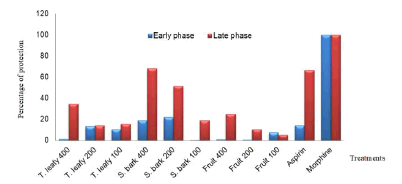
Figure 2: Percentage of protection of extracts against formalin-induced pain in rats. T : Twigs, S : Stem
According to hot water tail immersion test
Table 3 and Figure 3 represent the data obtained in the test of immersing the tails of the rats in hot water. At 60 minutes after the treatment, the extract of the stem barks at 400 mg/kg significantly increased the time of removal of the tails of rats from hot water (6.40 + 0.45) by compared to the negative control batch (2.86 + 0.57). The percentage increase in withdrawal time was 20.7 %, at 60 minutes after treatment; the activity was lower at 90 minutes after treatment. Under the same conditions, morphine at a dose of 10 mg/kg was very active during the three times for measuring the tail withdrawal time (Table 3 and Figure 3).
Table 3. Time (in seconds) of withdrawal of the tail of the rats from hot water before and after the administration of the extracts
| Extract | Dose (mg/kg) | T = 0 | T = 30 minutes | T = 60 minutes | T = 90 minutes |
|---|---|---|---|---|---|
| N. control - | 3.74 + 0.61 | 3.90 + 0.68 | 3.85 + 0.86 | 3.54+ 0.66 | |
| Morphine | 10 | 3.96 + 0.87 | 13.57 + 1.47*** | 16.178 + 1.72*** | 11.83 + 1.33*** |
| Twig leafy | 400 | 3.36 + 0.93 | 3.65 + 0.47 | 4.01 + 0.85 | 3.72 + 0.65 |
| 200 | 3.35 + 0.45 | 4.56 + 0.63 | 4.34 + 0.77 | 3.36 + 0.76 | |
| 100 | 2.81 + 0.59 | 3.30 + 0.81 | 4.43 + 0.91* | 3.08 + 0.44 | |
| Sem bark | 400 | 2.86 + 0.57 | 3.60 + 0.8 | 6.40 + 0.45*** | 4.41 + 0.35* |
| 200 | 4.31 + 0.84 | 5.52 + 0.85 | 5.1 + 0.76 | 5.33 + 0.97 | |
| 100 | 3.13 + 0.53 | 3.77 + 0.41 | 3.21 + 0.39 | 3.28 + 0.7 | |
| Fruit | 400 | 2.88 + 0.5 | 3.26 + 0.39 | 3.88 + 0.55 | 3.35 + 0.49 |
| 200 | 4.46 + 0.73 | 3.96 + 0.69 | 5.43 + 0.7 | 4.31 + 0.37 | |
| 100 | 3.01 + 0.78 | 3.47 + 0.49 | 3.77 + 0.51 | 3.52 + 0.76 | |
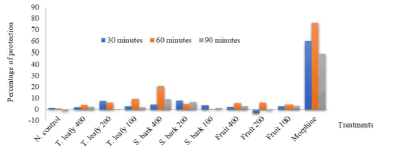
Figure 3: Percentage of protection of extracts against pain caused by immersing the tails of rats in hot water. N : negative, T : Twig, S: stem
Anti-inflammatory activity
Most of the extracts significantly reduced carrageenan-induced inflammation as shown in . Stem barks presented the best inhibition percentages at all hours, respectively 58.57; 61.41 and 67.28% at 1 hour, 3 hours and 5 hours after injection of carrageenan. Under the same conditions, aspirin gave as percentages of activity 42.55; 77.59 and 82.10% (Figure 4)
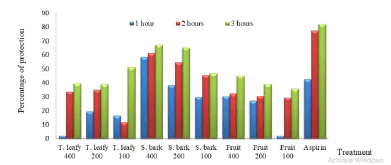
Figure 4: Percentage of protection of extracts against carrageenan-induced edema in rats. T: Twig, S: Stem
The objective of this work was to evaluate the analgesic and antiinflammatory activity of infused leafy twigs, trunk bark and fruit of A. nilotica. Preliminary assessment of the acute toxicity of the extracts showed that they are not toxic up to 5000 mg/kg. Therefore, according to the globally harmonized OECD classification system, these extracts can be classified as category 5 and considered to be of low oral toxicity. These results are in agreement with those reported in the literature [29,30]. These data are in favor of safe use of infused leafy twigs, stem bark and fruits of A. nilotica per os.
Three tests were used to assess analgesic activity: the writhing test, the formalin test and the tail immersion test. Intraperitoneal injection of acetic acid induced numerous abdominal cramps resulting in stretching movements (writhing) in mice (Table 1). The extracts, in particular those of stem bark, significantly reduced the number of abdominal cramps in mice. A percentage of pain inhibition similar to that of paracetamol was observed with the stem bark extracts at 400 mg/kg (Figure 1). The decrease in the number of writhings by the extracts suggests that they possess peripheral analgesic effects. The analgesic action of the extracts could go through the inhibition of the synthesis of prostaglandins by action on cyclooxygenases (COX). Indeed, pain induced by acetic acid is sensitive to NSAID such as aspirin and paracetamol [31,32,33] which are peripheral analgesics with COX inhibition as action mechanism.
Only stem bark extracts significantly reduced the licking and biting time of the paw injected with formalin during the early phase. The pain induced in the early phase by formalin is of neurogenic origin and would result from the direct stimulation of nociceptors [34]. This suggests that these extracts exhibit central analgesic activity. All other extracts protected against formalin-induced pain during the late phase with dose-dependent activity with stem bark. This protection suggests that all the extracts have peripheral analgesic properties because during this late phase the pain induced is pain of peripheral origin during which inflammatory phenomena would occur [34]. This pain is sensitive to NSAID. So, like NSAID, extracts could also inhibit prostaglandin synthesis.
In the tail immersion test, none of the extracts showed significant activity at the 30th minute. At the 60th and 90th minutes, the stem barks significantly increased the immersion time of the tails of the rats in hot water. This suggests that these extracts also exhibit central analgesic activity. The central analgesic activity was greater at the 60th minute after the administration of the extracts. This confirms the central analgesic activity exhibited by the stem barks in the early phase of the formalin test.
The administration of carrageenan induced inflammation in the rats as could be seen through the increase in the volume of the paws of the rats of the negative control group. All extracts significantly reduced edema in rats with the best stem bark activity. This suggests that the extracts exhibit antiinflammatory properties. The anti-inflammatory activity of the extracts was more marked at 3 and 5 hours. During the last phase (2h to 6h after administration) of carrageenan-induced inflammation, the mediators involved are prostaglandins [35]. The anti-inflammatory activity of the extracts could therefore go through the inhibition of the synthesis of prostaglandins. In a recent study, extracts of leafy twigs, stem barks and fruits of A. nilotica exhibited free radical scavenging properties [36]. This may partly explain the anti-inflammatory mechanism of action of plant extracts. The polyunsaturated fatty acids of membrane phospholipids are the targets of certain free radicals, thus initiating chains of lipid peroxidation [37]. This phenomenon will result in the blocking of the anti-inflammatory process and partly elucidates the involvement of free radicals in the inflammatory phenomenon. The antiradical activity of A. nilotica extracts could therefore also contribute to their analgesic and anti-inflammatory effects.
The results thus obtained are similar to those of other studies which have also shown the analgesic and anti-inflammatory activity of extracts and molecules isolated from Acacia nilotica. Methanolic extracts of stem bark and its various fractions significantly inhibited acetic acid-induced pain in mice [38]. The methanolic extract of stem bark was also effective against formalin-induced inflammation in rats [16]. Niloticane, a diterpene isolated from the ethyl acetate extract of the stem bark of A. nilotica showed strong prostaglandin inhibition activity (percentage inhibition >75% on COX 1 and <40% on COX 2) [17]. Aqueous maceration of the seeds at 500 mg/kg caused a 20 % reduction in carrageenan-induced inflammation in the paws of rats. The same extract also produced a reduction in lag time (hot plate response) [39]. These analgesic and anti-inflammatory properties show that A. nilotica extracts can greatly reduce pain and inflammation associated with liver disease.
This study confirmed the analgesic and anti-inflammatory properties of non-toxic infused leafy twigs, stem bark and fruits of A. nilotica.
These analgesic and anti-inflammatory properties of aqueous extracts of A. nilotica, in particular the stem bark, can contribute to the management of painful and inflammatory symptoms associated with liver disease.
This work benefited from the financial and material support of the Training of Trainers Program (TTP) of the government of Mali obtained through the University of Sciences, Techniques and Technologies of Bamako (USTTB).
[Google Scholar] [Cross Ref]
[Google Scholar] [Cross Ref]
[Google Scholar] [Cross Ref]
[Google Scholar] [Cross Ref]
Citation: Sekou D. et al. Analgesic and Anti-Inflammatory Activity of Acacia nilotica L. Wild. Ex delile (Mimosaceae), used in the Traditional Management of Liver Diseases in Mali. Nat Prod Chem Res. 2023, 11(1), 1-5.
Received: 17-Feb-2023, Manuscript No. NPCR-23-21826; Editor assigned: 21-Feb-2023, Pre QC No. NPCR-23-21826(PQ); Reviewed: 25-Feb-2023, QC No. NPCR-23-21826(Q); Revised: 26-Feb-2023, Manuscript No. NPCR-23-21826 (R); Published: 01-Mar-2023, DOI: 10.35248/11.2.1-5
Copyright: ©2023 Sekou D. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC-BY, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.