Research Article - (2020) Volume 11, Issue 9
Background: We aimed to determine the feasibility of using an established telehealth system to monitor potential mood changes in individuals with type 2 diabetes when commencing an injectable glucose-lowering therapy.
Methods: Individuals with type 2 diabetes commencing an injectable therapy as part of their routine clinical care when not achieving glycemic control on oral antidiabetic therapy, or when instituted as part of a randomized clinical trial, were asked to self monitor their mood for six months. Participants were asked to complete the Quick Inventory of Depressive Symptoms–Self Report (QIDS-SR) weekly and the Diabetes Distress Scale (DDS17) monthly.
Results: Seven participants were recruited who all initiated an injectable therapy and were followed up for median 33 (27–37) weeks. Median (IQR) proportions of returned QIDS-SR and DDS17 questionnaires were 100% (86%–100%) and 100% (100%–100%) respectively, with completeness 88% (80%–100%) and 100% (100%–100%). DDS17 scores increased (worsened) during the first month after injectable therapy initiation, followed by a gradual decline in QIDS-SR scores and stabilizing DDS17 responses.
Conclusion: We show that an online self-management system can be used to monitor mood remotely in type 2 diabetes population, and could provide additional metrics to help inform diabetes management.
Tele-health; Diabetes management; Injectable therapy
Mood disturbance in people living with diabetes occurs twice as commonly as in those without diabetes [1]. Low mood impacts negatively on an individual’s ability to self-care for their diabetes [2], and is associated with an increased likelihood of developing cardiovascular disease [3]. Developing strategies that better identify changes in mood in people with diabetes could offer the chance to intervene with appropriate support at an earlier stage, this may have important benefits for both quality of life but also for long term complications if it facilitates self-management and supports concordance with medication and complication screening.
The aim of our study was to determine the feasibility of using an established telehealth system to monitor potential mood changes in individuals with type 2 diabetes (T2D) when commencing an injectable glucose-lowering therapy.
Study population
The study was conducted at the Oxford Centre for Diabetes, Endocrinology and Metabolism (OCDEM) with participants screened and recruited via the OCDEM diabetes specialist outpatient clinics, neighbouring diabetes clinics and Primary Care. Inclusion criteria for all participants were: to have an established diagnosis of Type 2 diabetes but failing to achieve their glycaemic target on oral antidiabetic therapy; be able and willing to give informed consent for participation in the study; and comply with its requirements and to have been offered injectable therapy for the first time, by their treating physician as part of their routine clinical care and independently of the study. The choice of insulin or GLP- 1 RA was determined by the participant in consultation with their treating physician.
The “True Colours” monitoring system
Participants were asked to monitor their mood using a remote mood monitoring system developed at the Department of Psychiatry, University of Oxford. The “True Colours” system is a technology assisted method of remote mood monitoring that has been pioneered in patients with bipolar disorder [4] in Oxford and is available online (https://oxfordhealth.truecolours.nhs. uk). The system generates weekly text/email alerts to participants to prompt them to register responses to two established selfreport questionnaires, the Altman Self-Rating Mania scale [5] and the Quick Inventory of Depressive Symptoms–Self Report [6]. It securely records their responses and uses these to capture participants’ moods over sustained periods.
The system was adapted for use in people with T2D by replacing the Altman Self-Rating Mania scale with the 17-item Diabetes Distress Scale (DDS17) [7], whilst retaining the QIDS-SR. A full list of the QIDS-SR questions is available online [8].
These data will then be used to determine the feasibility of remote mood monitoring in type 2 diabetes, the prevalence of mood symptoms in individuals with type 2 diabetes initiating injectable therapy and whether there is a differential effect on mood between the two types of injectable therapy. All participants had no concurrent or past psychiatric care and their laboratory results for liver, renal, thyroid function and lipid profile were normal. The study protocol was approved by the West Midlands- Coventry & Warwickshire Research Ethics Committee, the study had been conducted in full conformity with relevant regulations and with the ICH Guidelines for Good Clinical Practice (CPMP/ ICH/135/95), July 1996 and subsequent amendments and in accordance with principles of the declaration of Helsinki. All study participants provided written informed consent [9-12].
Study design and measurements
On the day of their scheduled procedure at the OCDEM Clinical Research Unit, following measurement of their vital signs, resting pulse rate (min-1) and blood pressure (mmHg), participants received a written summary of the mood monitoring questionnaires and instructions on how to complete them and return their responses. All participants had also undertaken a brief supervised completion of their first 16-item QIDS-SR and first 17-item DDS17 and submitted the data, thus having the opportunity to query the process and have any technical issues resolved. With prior consent from each participant, routine clinic data collected during three further routine NHS clinical care provider appointments were used for this research study. The clinical site team did not attend these visits, but referred to the participant’s medical notes or patient held records as source data following each visit.
The second study visit had been ideally scheduled within three months of visit 1, when the participant had attended the usual care provider for initiation of their injectable therapy. Following visit 2, the research team contacted the participant by e-mail or SMS to confirm the start date for their injectable therapy and also to confirm the type of therapy prescribed. They also enquired whether the participant has experienced any Serious Adverse Events. A follow up visit (visit #3) was then ideally scheduled at 3 months after initiation of injectable therapy and they were asked to have a blood sample for the measurement of HbA1c taken, with measurement of body weight, BMI, blood pressure, concomitant medication(s) and Serious Adverse Events recorded as well, as part of routine clinical care. At 6 months after initiation of injectable therapy, the closeout visit (visit 4) was scheduled.
Primary and secondary endpoints / outcome measures The primary endpoint of this study was the feasibility assessment of using the questionnaires, based on 1) the number of participants completing the study; 2) the number of ‘completed’ questionnaires (which had been submitted during the study with one or more answer(s) missing); 3) the number of ‘correctly completed’ questionnaires, i.e., those which had been submitted during the study with answers provided for all questions. Secondary outcome measures were: changes in weekly 16-item QIDS-SR following initiation of the chosen injectable therapy, changes in monthly 17- item DDS17 following initiation of the chosen injectable therapy and changes in HbA1c, measured as part of the routine clinical care, prior to and three & six months after initiation of the chosen injectable therapy.
Clinical safety of study participants
This had been a non-interventional study administering questionnaires for quantitative analysis. The study did not involve any treatments or interventions but collected self-reported information from patients who were being administered an injectable therapy as part of usual care. Regular monitoring of mood and distress was an augmentation of usual care, i.e. reflected an increase in quality of care from that which would typically be provided to such patients. Since depression and diabetes-related distress are common in patients with diabetes, it was important that appropriate procedures had been in place to monitor and respond to scores outside the normal range. The following management plan based upon the principles of management of depression specified in the latest NICE guidelines (2009, CG919), was implemented to ensure all individuals were supported fully throughout their participation. These principles include: 1) being alert to possible depression, and asking appropriate screening questions (NICE 1.3.1.1); 2) if the screen is positive, refer to an appropriate professional (typically GP) for further assessment (NICE 1.3.1.2. and 1.3.1.3); 3) using a validated measure (e.g. QIDS-SR) to inform and evaluate treatment (NICE 1.3.1.4); 4) referral for urgent further assessment those who might present ‘considerable immediate risk to themselves’ (NICE 1.3.2.1). Appropriate ‘trigger’ thresholds for the QIDS-SR and DDS-17 questionnaires had been agreed by the clinical team prior to the start of the study and were reviewed at 6 weeks and 4 months into the study. All questionnaire responses were regularly reviewed within one week of receipt by the study research nurse or Investigator. If a trigger threshold was exceeded for the first time, this information was communicated to the patient's GP and to the patient within one week of becoming aware, giving simple written information on their questionnaire scores and their interpretation. If the threshold was passed on further occasions but the participant’s questionnaire scores remained the same, then this information was communicated monthly to the patient and their GP. However, if the participant’s scores worsened, then this information was communicated to the patient's GP and to the patient within one week of becoming aware. An escalated contact was urgently undertaken for responses of either ‘2’ or ‘3’ to question 12 of the QIDS-SR questionnaire (‘Thoughts of death or suicide’). In this instance, the Investigator telephoned the participant within 24 hours of becoming aware to determine whether the individual requires urgent assessment by their GP or by psychiatric services.
All data were captured electronically and scores automatically calculated. Scores surpassing trigger thresholds were easily identifiable by study staff meaning review of scores from multiple participants was rapid and largely automated.
The DDS yields a total diabetes distress score and four sub scale scores: emotional burden, physician-related distress, regimenrelated distress and interpersonal distress). A mean item score of greater than or equal to 3 is classed as moderate distress worthy of clinical attention.
Total scores for QIDS-SR range from 0-27. Scores equate to severity of depression with a score of 0-5 indicating no depression, 6-10 mild depression, 11-15 moderate depression, 16-20 severe depression and 21-27 very severe depression.
Data processing
Study data were entered directly at site into a fully validated ICH GCP compliant Electronic Data Capture (EDC) Trial Management System (TMS) (MACROTM, Infermed Ltd., London, UK; version 3.0.83), using established streamlined EDC technology. Real time data from the monitoring system were transferred to the electronic database designed and managed by the University of Oxford True Colours Team, Department of Psychiatry, Warneford Hospital, Oxford, which maintains confidential NHS-approved data-storage systems for pseudo-anonymised patient data for analysis. The mapping from confidential patient data to study ID was kept within the premises of the clinical site.
Statistical Analysis
Demographic and clinical data were summarised using appropriate measures of central tendency and dispersion, or frequency and percentage (%). The primary endpoint (frequencies of completing participants and correctly completed questionnaires over time), as well as the actual responses to the QIDS-SR16 and the DDS17 questionnaires were tabulated, summarized and reported overall, by type of injectable therapy and by time. For the statistical summaries and graphical representations, both QIDS-SR16 and the DDS17 questionnaires were aligned for the individual subjects. The first observation in both, before treatment incitation we regarded as pre measurement observation while observations taken after the treatment initiation were post treatment observations. A 4-Week Moving Average (MA) and 4-Week Exponential Weighted Moving Average (EWMA) methods were employed to identify response trends over time for the QIDS-SR16 questionnaire responses. To obtain a similar graphical representation for the DDS17 questionnaire monthly trend, both 4-Week MA and 4-Week EWMA were also employed by “blowing up” the monthly data, repeating each monthly data 4 times on a weekly basis to create a balanced panel alignment with the weekly collected data. All analyses were considered as exploratory with no adjustment for multiplicity. Exploratory statistical significance was considered with a p-value of 0.05. It was assumed that for the primary endpoint to be successful, 80% of participants would have completed at least 80% of the two questionnaires, based on previous experience with remote mood monitoring studies. There are no prior data on effect sizes in the population studied, therefore, no formal sample size calculation was undertaken.
Participant characteristics
Seven consented individuals (four male and three female) were recruited. Median (IQR) age was 60 (49–65) years and diabetes duration 11 (2–14) years. 71% of participants were active smokers, 57% had a family history of diabetes and 29% had a family history of depression. One female participant, who eventually commenced exenatide, had been on low dose sertraline therapy from her GP but never under secondary psychiatric care. Six participants were receiving metformin, five a sulfonylurea and four a dipeptidylpeptidase (DPP)-IV inhibitor, with four participants under triple therapy and two more under maximised double oral antidiabetic treatment. One participant commenced insulin therapy directly.
Injectable therapies
Four participants initiated insulin and three various GLP-1 receptor agonists titrated over time and were followed up for a (median; IQR) period of 33 (27,37) weeks. All three participants commencing a GLP-1RA received a different agent: one participant initiated exenatide extended-release once weekly, which remained unaltered until the study close-out, whereas one other participant was started on taspoglutide 10mg once weekly, titrated to 20mg once weekly at his 2nd visit and a third one initiated liraglutide 0.6mg once daily which was titrated to 1.2mg once daily at his 2nd visit. No specific information is available regarding the exact insulin formulation and type (basal or prandial) administered to the four participants initiating insulin therapy.
Questionnaire responses and scoring
The median proportions of participants returning their QIDS-SR and DDS17 questionnaires were 100% (86%–100%) and 100% (100%–100%) respectively, with questionnaire completeness of 88% (80%–100%) and 100% (100%–100%), respectively. DDS scores increased (worsened) consistently before and during the first month after initiation of injection therapies with lower values thereafter and stable QIDS scores (Figure 1). More complete data were returned for the monthly versus the weekly questionnaires. Missing data tended to reflect the return of a questionnaire with some items not answered rather than a participant not returning a questionnaire at all.
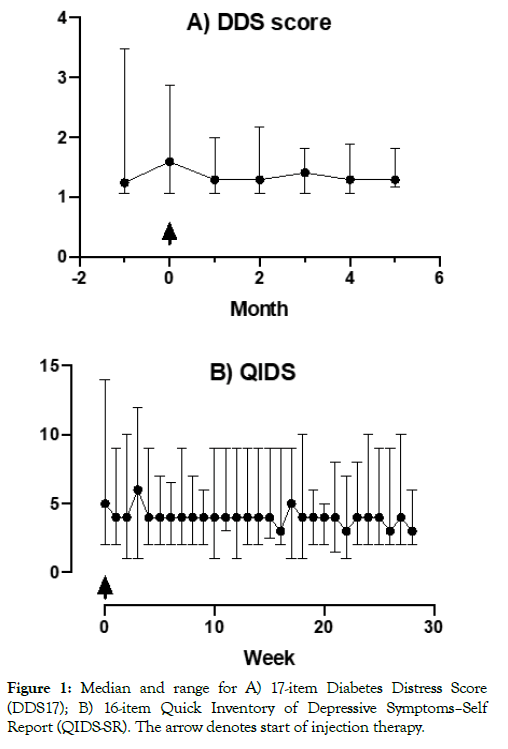
Figure 1: Median and range for A) 17-item Diabetes Distress Score (DDS17); B) 16-item Quick Inventory of Depressive Symptoms–Self Report (QIDS-SR). The arrow denotes start of injection therapy.
Laboratory and other data
Regarding the HbA1c values, data were available from only two participants showing a decline from 90 to 61mmol/mol and from 98 to 44mmol/mol from initiation of injectable therapy to study close-out, respectively.
Our study is limited by a number of factors that need to be considered when evaluating the role the “true colours” system may have in supporting the identification of mood changes in people living with type 2 diabetes. We originally set out to recruit 20 people with type 2 diabetes needing to start injectable therapy yet were only able to recruit seven. This occurred in spite of determined recruitment efforts. Poor recruitment may in part represent a change in the delivery of diabetes care such that increasingly injectable therapies are initiated in primary care so fewer of this patient group are seen in specialist services. Another explanation may be that this technology or the intensity of monitoring required deterred people from taking part. It may also be as a result of people with diabetes not recognising the potential impact that type 2 diabetes and its treatments can have on mood or the more general social stigma of mental ill health12. The small sample size also makes it impossible to draw any meaningful conclusions about the differential effect of different injectable therapies on mood. We had suspected that the impact of GLP-1 RAs on mood may be less than that of insulin because they are associated with weight loss but further studies will be needed to determine if this is indeed the case.
More complete data were returned for the monthly versus the weekly questionnaires. This may indicate that monthly data return is more acceptable and feasible for people than weekly or it may reflect a preference to reply to the questions asked in the DDS over the QIDS-SR. Further work could examine this in more detail.
A strength of this small study is that we were able to detect subtle changes in mood associated with initiating an injectable therapy for type 2 diabetes for the first time. DDS scores were consistently increased during the first month after injectable therapies commenced, driven primarily by emotional burden and regimenrelated distress. QIDS scores were stable throughout the study.
These findings have immediate clinical applicability. Whilst many would agree that people with diabetes often find the initiation of insulin stressful for a variety of reasons including fear of weight gain and hypoglycaemia and a feeling that they have “failed” in the management of diabetes many would not consider that starting GLP-1 RA therapy was similarly stressful perhaps because of the ability of these agents to result in weight loss. Our findings support the need to be open and honest with people initiating injectable therapy or any kind and to counsel them about the high likelihood of a transient impact on their mood not just for those initiating insulin therapy.
The ability to find innovative and cost-effective solutions to better support people living with diabetes and other long-term conditions is a key focus of NHS England at present. The NHS is looking for scalable digital health solutions with a common approach across disease areas (health call reference). Type 2 diabetes has been identified as offering a particular challenge in ensuring that people understand their condition and are able to confidently self-manage their condition (test beds reference). The ability to demonstrate that the “True Colours” system, developed for use in one long term condition (bipolar disorder) but equally applicable to diabetes and therefore, potentially many other long term conditions is an appealing prospect. The use of such a system would allow large scale delivery of mood monitoring with a central review of the data captured and the ability for prompt intervention. The ability for the data captured via “True Colours” to be embedded in electronic patient records offers further appeal. At present such a comprehensive and regular assessment of mood in diabetes is not possible. The 2 question abbreviated diabetes distress score was previously used to screen for depression in people with diabetes and was remunerated in primary care through the quality and outcomes framework. However, this instrument has since been found to be unreliable and is no longer a QOF indicator in people with diabetes. Our study, whilst small, has demonstrated that the “true colours” system is acceptable to patients and able to identify subtle changes in mood associated with starting injectable therapy. It is possible that the “true colours” system may be better placed rather than to identify mood around stressor events such as starting an injectable therapy but for long term monitoring of mood over many years to better identify the development of depression than the previously used DDS2 questions. Further studies could examine this.
This proof-of-concept study shows that an online self-management system can be used to monitor mood remotely in people with Type 2 diabetes, and could provide additional metrics to help guide diabetes management. Further studies could examine if there is further value that this technology could have in monitoring mood in people living with type 2 diabetes.
Funding statement
‘This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors’
Disclosures
None
Author’s contribution
The authors contributed equally to the conception and design of the work, the acquisition, analysis, and interpretation of data for the work. In addition all authors equally drafted the work, revised and approved the version to be published. The authors all agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
We thank MJ Theodorakis and JT George for their help in conducting the study.
Citation: Agbaje OF, Holman R, Price H, Price J, Goodwin G, Geddes J (2020) A Proof of Concept Study to Evaluate the Impact of An Established Telehealth Mood Monitoring System on Mood in Individuals with Type 2 Diabetes when Initiating An Injectable Therapy. J Diab Metab 11:857.
Received: 11-Jun-2020 Published: 28-Oct-2020
Copyright: © 2020 Agbaje OF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.