Case Report - (2022) Volume 11, Issue 3
Breast augmentation surgery with an injection of free liquid silicone has been performed since the early 1960s but was abandoned by most practitioners because numerous complications have been reported, such as migration of silicone to other parts of the body, inflammation, discoloration, and the formation of granulomas, ulceration and fistulae, anaplastic large cell lymphoma, silicone-induced granuloma, breast carcinoma. In this case report, we describe an invasive breast carcinoma, diagnosed via core needle biopsy, in breast augmentation by injecting free liquid silicone ten years ago, including breast imaging findings on mammography and Magnetic Resonance Imaging (MRI), macroscopic and microscopic features. The nipple-sparing mastectomy was performed for two breasts that were injected with liquid silicone. In the right breast with adenocarcinoma, surgical margins above nipple-areolar were assessed intra-operatively by frozen section before immediate breast reconstruction was carried out in the same operation. Traverse Rectus Abdominal Muscle (TRAM) flap procedure reconstructed for right breast and implant breast reconstruction for the left breast. We received amazing post-operative results.
Breast Cancer • Liquid Silicone Injection • Breast Reconstruction
Breast augmentation surgery with injection of free liquid silicone has been performed from the early 1960s but was abandoned by most practitioners after a 1969 publication described multiple long-term adverse effects [1]. The procedure consists of injecting medical or, in many cases, non-medical grade liquid silicone into the retromammary space, between the pectoralis major muscle and the fibro glandular breast tissue component. Despite multiple known associated complications, the procedure remains available in parts of Asia, Eastern Europe and South America largely owing to its low cost [2].
The early results of injections were often quite acceptable. The complications that followed these injections frequently did not show up until five or 10 years later. However, five or six years later, they developed ugly knots and draining fistulae that required surgical intervention. These complications ranged from aesthetic failure to death. They included pulmonary embolism, migration, ulceration, fistulae, infection and necrosis. These complications would frequently lead to breast amputation even breast cancer [3].
We report a breast cancer case in women performing breast augmemtation with free liquid silicone injection.
Patient (patient) female, 63 years old, 2 months ago she felt a hard mass in her right breast. The patient came to a specialized hospital in Hanoi and was examined by ultrasound and mammography for the rightbreast tumor BIRADS 4. Then, the patient performed a core needle biopsy to be diagnosed histopathologically and received the result “Invasive ductal carcinoma, histological grade 1”. The patient entered Ho Chi Minh City with the desire to remove the tumor and reconstruct the breast. History: Patient had liquid silicone injection into 2 breasts 10 years ago, performed at a private beauty salon.
At EMCAS plastic surgery hospital, patients are examined and completed all general tests, pre-surgery, and tumor assessments. Clinical examination showed that the right breast tumor was about 2x3cm in size, located at 11 oclock position , about 2cm from the upper edge of the areola, solid density, not mobile, boundaries unknown, skin above tumor shows no adhesions with underlying tumor tissue, no image (Figure 1).
Figure 1: Breast apperance after injection the liquid silicone 10 years
The surgery of choice for this patient is total bilateral mastectomy, with nipple conservation, combined with reconstruction of right breast tissue by TRAM flap - transverse rectus abdominis and left breast with the breast implant in the same time . During resection of the tumor-containing right mammary gland, the retained upper margin of the areola is sent for immediate biopsy to assess the safety of the surgical margin. Bilateral mammary gland tissue along with level 1 and level 2 lymph nodes in the right breast was taken and sent for histopathology and immunohistochemical staining with markers ER, PR, HER-2, Ki67, and p53 to guide treatment, follow-up after surgery for the patient. The macroscopic image of the right breast tissue containing the tumor shows that the tumor is located quite close to the fascia below the tumor but has not invaded down to this layer, about 1.5cm from the skin above the tumor. The tumor has the largest diameter of the tumor about 2.5cm, the border is not clear with the surrounding breast tissue, ivory white, heterogeneous. The surgical margins and the fascia just below the tumor were resected to see if tumor cells were still present. The left breast tissue showed no gross lesions. Dissected 12 lymph nodes at level 1 and 2 lymph nodes at level 2.
No skin or orange skin contractions were seen on the right breast. Mammography (Figure 2a,b) and MRI (Figure 3) Breast results: Both breasts have injected/implanted liquid silicone to create scattered patches and patches in the subcutaneous fat tissue of the breast and outside of the mammary gland. The right breast has a lesion occupying the position of the upper outside, d#40x28x48mm, irregular spine margins, strong contrast enhancement, poor homogeneity, and late discharge. The lesion has not invaded the chest wall muscles and does not pull on the skin of the breast and nipple. There was no focal lesion in the left breast. No enlarged lymph nodes were seen in the mammary gland tissue or bilateral axillary fossa. The results of Computed Tomography (CT) scan of the entire skull, neck, chest, abdomen, and pelvis did not reveal any lesions or suspected lymph node metastasis.
Figure 2: Anterior film mammogram (2a) and Lateral film (2b) bilateral mammogram
Showing found a tumor in the right breast with irregular margins, heterogeneous density, The calcified nodules in the left breast tissue have rounded edges, and uniform density. Bilateral breast tissue with a fibrous appearance almost entirely
Figure 3: Breast MRI image.
The right breast has a mass lesion, irregular spine margins, strong contrast, and poor homogeneity. The lesion has not invaded the chest wall muscles and does not pull on the skin of the breast and nipple. There was no focal lesion in the left breast.
The surgery of choice for this patient is total bilateral mastectomy, with nipple conservation, combined with reconstruction of right breast tissue by TRAM flap - transverse rectus abdominis and left breast with the breast implant in the same time . During resection of the tumor-containing right mammary gland, the retained upper margin of the areola is sent for immediate biopsy to assess the safety of the surgical margin. Bilateral mammary gland tissue along with level 1 and level 2 lymph nodes in the right breast was taken and sent for histopathology and immunohistochemical staining with markers ER, PR, HER-2, Ki67, and p53 to guide treatment, follow-up after surgery for the patient. The macroscopic image of the right breast tissue containing the tumor (Figure 4) shows that the tumor is located quite close to the fascia below the tumor but has not invaded down to this layer, about 1.5cm from the skin above the tumor. The tumor has the largest diameter of the tumor about 2.5cm, the border is not clear with the surrounding breast tissue, ivory white, heterogeneous. The surgical margins and the fascia just below the tumor were resected to see if tumor cells were still present. The left breast tissue showed no gross lesions. Dissected 12 lymph nodes at level 1 and 2 lymph nodes at level 2.
Figure 4: Right breast tissue after resection seen from the anterior (A), side (B), and transverse views of the tumor (C)
Microscopic results (Figure 5), right breast tumor is invasive breast carcinoma, chromosome, grade 2 (according to Bloom-Richardson system). The left breast tissue showed only fibrosis. All 12 lymph nodes in level 1 and 2 lymph nodes in level 2 have no metastases. The surgical margins above, below, inside, outside, and below the tumor fascia were all free of tumor cells. Immunohistochemical staining results (Figure 6) with 5 markers gave the following results: ER (0/8 points), PR (2/8 points), HER-2 (3+), Ki67 15%, p53 (+).
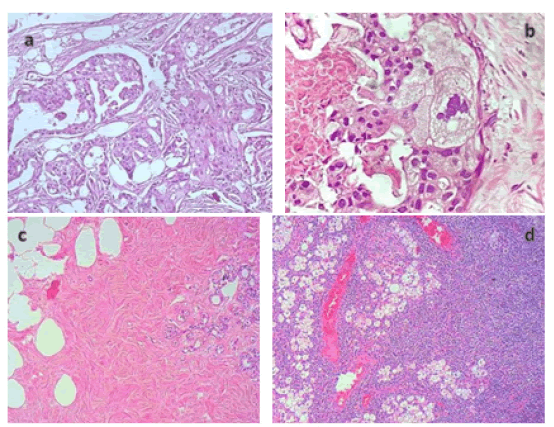
Figure 5: The tumor tissue in the right breast shows the image of tumor cells lined up, mostly ducts are still formed, nuclei are malformed, basophilic, nucleus/cytoplasmic ratio >1, many abnormal cell divisions often, interspersed are foci of tumor cells with abundant cytoplasm, foamy cells (a, b). The left breast tissue on the right and left breast tissue show fibrosis (c). The interior of the lymph node showed histiocytic tissue with many vacuoles, consistent with the clinical picture of silicon lymphadenopathy (d).
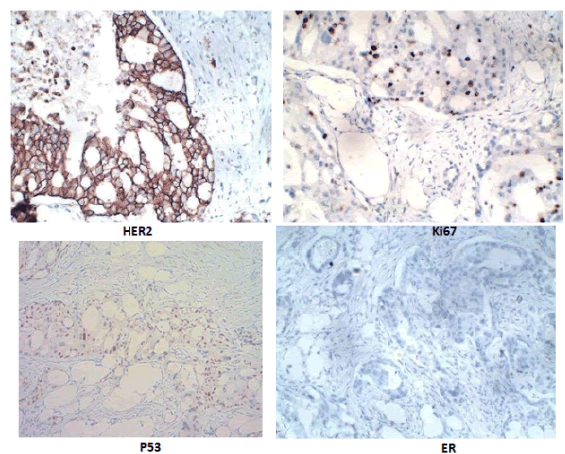
Figure 6: Immunohistochemical staining results with 5 markers: HER-2 (3+), Ki67 15%, p53 (+), ER (-).
After surgery, the patient's general condition was good, the sutures were dry and clean, the size of the mammary glands on both sides was relatively equal (Figure 7), the TRAM flap was well perfused, and the patient's abdominal wall was significantly reduced. . After surgery, the patient is monitored and will continue to receive chemotherapy and targeted therapy according to the breast cancer treatment protocol of the Ministry of Health [1].
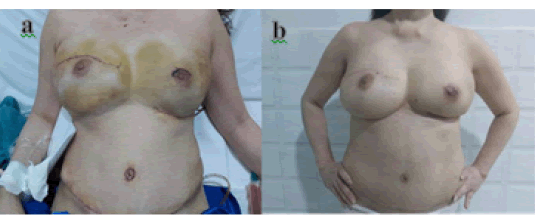
Figure 7: Photos after surgery 6 days (a) and after 1 month (b).
Micrograph of tumor typical for invasive breast carcinoma, with apocrine variant (Figure 5a-d) with images of tumor cells with clear cytoplasmic border, cytoplasm containing many vacuoles that resemble foam cells, or histiocytosis, nuclei are atypical, eccentric, nuclear margins are irregular, chromatin rough, nuclei clear or hyperchromatic. This is also a rare variant in invasive breast cancers, with an incidence of 1-4% [4]. With this variant, the prognosis of the tumor is comparable to or better than that of ductal carcinoma of the breast intrusion [4,5]. With a measured tumor size of #2.5cm, no lymph node, and distant metastases, it should be classified as stage IIA (T2N0M0) according to the 8th TNM classification of the UICC (Union International Contre le Cancer) and AJCC (American Joint Committee on Cancer) 2017 [1]. The left breast tissue on the right and left breast showed almost total fibrosis, and the cysts in the level 1 lymph nodes showed typical silicon-induced images on the mammary gland tissue and draining lymph nodes (Figure 8).
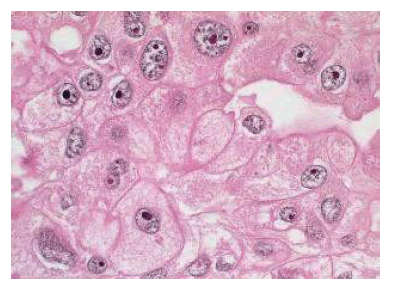
Figure 8: Breast carcinoma, apical differentiation [7]
ccording to the guidance of the Ministry of Health of Vietnam, cases of mastectomy can be considered for breast reconstruction surgery if the patient has a need and oncology has no contraindications. Exotic materials or autologous dermal flaps can be used, or a combination of both. Breast reconstruction can be performed immediately during mastectomy or at some point after completion of treatment [1]. Therefore, with the assessment of the patient's general condition on clinical and subclinical, along with the location and size of the tumor, we chose to remove the entire breast tissue on both sides, with nipple preservation and the result. Combination of right breast tissue reconstruction with rectus abdominal flap (transverse rectus abdominis) and left breast with a breast implant in the same surgery. The skin margin above the areola was evaluated intraoperatively by a frozen section with results within 45 minutes, giving the surgeon peace of mind in the subsequent reconstruction. The introduction of the TRAM flap in 1982, introduced by Hartrampf et al., brought mammography into the modern era. This technique is a reliable autologous tissue transfer technique that transfers the flap from the lower abdomen to reconstruct the breast. This surgery also contributes to the reduction of the abdominal wall [6]. After surgery, the patient had immunohistochemical results of the Her2(+++) group
Although until now, the relationship between liquid silicone injection and breast cancer has not been determined, however, there have been many reports in the world of individual cases of breast cancer appearing in patients receiving breast augmentation by free liquid injections. For silicone breast implants, the FDA has warned of an association with Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL), which is a fairly rare cancer and is primarily associated with breast augmentation. Related to breast augmentation materials are texture implants. In the past, silicon was considered an inert substance, but there is a lot of evidence that it is an immunogenic substance [7]. According to author Eduardo Fleury et al., carcinoma may be the result of inflammation rather than inflammation in response to carcinoma. The author's hypothesis suggests that free silicon can induce metaplasia or dysplasia of mammary epithelial cells, which may vary by the body's response to foreign antigens, from well-differentiated tumors to undifferentiated [8,9] ,and we think that our
case could also be due to this mechanism. Although there is no definitive evidence on what causes this case of breast cancer, our report hopes to contribute to the database on the association of breast cancer with silicon in Vietnam in particular and the world in general.
Besides benign complications such as moving to other locations in the body, cyst formation, granuloma formation, and silicon can also be the cause of malignant lesions. Based on silicon as a substance that can induce an immune response expressed by transforming mammary epithelial cells, it is possible that these metaplastic and paraneoplastic cells have transformed into malignant cells. However, we need more data on this issue and systematic studies to draw firm conclusions for this hypothesis. Performing immediate breast reconstruction after nipple sparing mastectomy allows the patient to quickly return to a normal life.
[Google scholar] [Cross Ref]
Citation: Nguyen DT, et al. A Case Report of Breast Cancer In Breast Augmentation Surgery With Injection of Free Liquid Silicone. Reconstr Surg Anaplastol, 2022, 11(4), 07-09
Received: 18-Aug-2022, Manuscript No. ACR-22-18979; Editor assigned: 25-Aug-2022, Pre QC No. ACR-22-18979; Reviewed: 29-Aug-2022, QC No. ACR-22-18979ACR-22-18979; Revised: 05-Sep-2022, Manuscript No. ACR-22-18979; Published: 10-Sep-2022, DOI: doi.10.37532/22.11.3.7-9
Copyright: © 2022 Nguyen D. H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.