Research Article - (2021) Volume 9, Issue 6
Syzygium balsameum Wight is a shrubs or trees of Myrtaceae family. The present study reports on the based-on and isolation of a flavonoid 2',4'-dihydroxy-3',5'-dimethyl-6'-methoxychalcone from the leaves of Syzygium balsameum and evaluation of antioxidant and cytotoxic property. The compound is characterized by the spectroscopic analysis UV, IR, EIMS, 1H, 13CNMR, Cosy, HMBC and HSQC. The compound 2',4'-dihydroxy-3',5'-dimethyl-6'-methoxychalcone is first time isolated from the Syzygium balsameum wight and exhibited good antioxidant and cytotoxic property compare to the extract and the standard.
Syzygium balsameum, Myrtaceae, flavonoid 2',4'-dihydroxy-3',5'-dimethyl-6'-methoxychalcone
The plant has been the best source of remedies for curing a variety of disease. This is why medicinal plants have played a key role in the worldwide maintenance of health [1]. Natural products from plants, animals, and minerals are the basis for treating human diseases . Natural products and their derivatives have been recognized for many years as a source of therapeutic agents and structural diversity. Natural products have a wide range of diversity of multidimensional chemical structures and the utility of natural products as biological function modifiers. A large number of important modern drugs and most of the traditional medicines are derived from medicinal plants. Some secondary metabolites of plants and animal origins like alkaloids, glycosides, flavonoids, and polyphenols possess medicinal properties [2]. A good amount of research work has already been conducted on different species of Syzygium and showed several biological activities such as anticancer antioxidants, cytotoxicity, thrombolytic, antidiabetic, antidiarrheal, and antimicrobial activity. Literature review also exhibited that many secondary active metabolites isolated form the different species of Syzygium, which have different pharmacological activity [3-11]. Syzygium balsameum also known as Eugenia balsamea Wight is a Shrubs or trees of Myrtaceae family. Literature review found that the leaves of Syzygium balsameum have several phytochemical constituents such as alkaloids, glycosides, flavonoids, tannins, saponins etc. and exhibited good antioxidants, cytotoxicity, thrombolytic, antidiabetic, antidiarrheal, and antimicrobial activity [12]. That made us curious to undertake fractions, evaluate the biological properties and isolation of bioactive principle from the leaves of Syzygium balsameum.
General experimental procedures
UV spectra were recorded on a shimadzu (uv-1800) UV/vis spectrophotometer, IR spectra were obtained on a Nicolet Magna-IR550 infrared spectrophotometer. EIMS were obtained with a Micromass GCT instrument at 70 eV ionization energy. 1HNMR, 13CNMR and 2DNMR spectra were recorded on a Bruker 400MHz Elemental analysis data were obtained with a Chem 3D. Silica gel (60 –120 mesh) for CC and G-254 for analytical TLC.
Collection and Identification of the Plant
The leaves of the plant, Syzygium balsameum formerly known as Syzygium nervosum were collected from the forest range of Bhabanipur, Gazipur, Dhaka, Bangladesh. It was identified by using standard taxonomical methods at the Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh. The accession number 47319. The plant is distributed throughout India, Myanmar, Bhutan, China and Thailand. In Bangladesh, it is found in the districts of Dhaka, Gazipur, Sylhet, and Chittagong.
The cleaned leaves were dried in the shade for 7 days followed by drying in an oven at 40°C for 2 hours. The leaves were then reduced into a coarse powder with the help of a mechanical grinder. The maceration technique was applied for the extraction process 400g of the dried powder of the leaves of Syzygium balsameum was taken in a jar containing desired solvent with continuous stirring with a magnetic stirrer for seven days and changed the solvents every 48 hours. The powder was first defatted with n-hexane for seven days. The defatted powder was then successively extracted with ethyl- acetate and methanol as before, each for 7 days. The solvents were removed using a rotary evaporator at 40°C. The extracts thus obtained were separately collected. Extracts are denoted as SBH, SBEa, SBM for n-hexane, ethyl acetate and methanolic extract.
The solvent of the ethyl acetate extract was removed from the extract (3000mL) on a rotary evaporator and kept in the desiccator for drying. The reddish black solid was 16.79 gm (SBEa), it was completely soluble in ethyl acetate. TLC (Thin Layer Chromatography) examination of SBH, SBEa, SBM showed complex mixture of different natural constituents. From the three extract the SBEa showed better resolution. Then 5gm of SBEa was partitioned with a silica gel column chromatography gradient mixture of (n-hexane: ethyl acetate) and (ethyl acetate: methanol) with increasing polarity which afford to eighteen fractions (SBEa E1-E18). TLC examination of all fractions showed preliminary separation of the extracts. From these fraction E4 showed better resolution in TLC examination. Fraction E4 (yellowish white solid 126mg) was further eluted with a silica gel column chromatography led to the isolation of a pure compound E4A yielded to 40mg orange-yellow solid, soluble in ethyl acetate, chloroform, methanol, etc. The compound is characterized as 2',4'-dihydroxy- 3',5'-dimethyl-6'-methoxychalcone which is first time isolated from the leaves of Syzygium balsameum.
Yellow solid; UV(CH3OH) λmax 339 and 225 nm; IR (neat) 3420(-OH), 3082, 3059, 3026 (aromatic C-H), 2854, 2927 (CH), 1628 (C=O), 1610, 1541, 1497 (C=C), 1167, 1111, 1072 (C-O) cm-1; 1HNMR (CDCl3) δ13.61 (1H; s, O-H at C-2'), 8.02 (1H; d; J=15.6 Hz, α-H), 7.86 (1H; d; J=15.6, Hz β-H), 7.44-7.42 (3H; m, H-3, H-4 &H-5), 7.67 (2H; J=6.0 and 12.0 Hz, H-2 & H-6), 5.44 (1H; s, O-H at C-4'), 3.68 (3H; s, -OCH3 at C-6'), 2.18 (3H; s, -CH3 at C-5'), 2.15 (3H; s, -CH3 at C-3'); 13CNMR (CDCl3) δ 193.4 (C=O), (162.0 (C-2'), 159.2 (C-4'), 158.9(C-6'), 142.9 (β- C), 135.4 (C-1), 130.2(C-4), 128.9 (C-3&C-5), 128.4 (C-2&C-6), 126.7 (α-C), 109.1(C-5'), 108.8 (C-1'), 106.5 (C-3'), 62.3 (-OCH3), 8.20 (CH3), 7.5 (CH3);MS(m/z) 298.1(M+).
Total flavonoid content
The total flavonoid content in plant extracts was determined using the colorimetric method involving aluminum chloride [13]. Quercetin was used as a reference standard and flavonoid contents of extracts were calculated as mg of QE/g of extract (Figure 1).
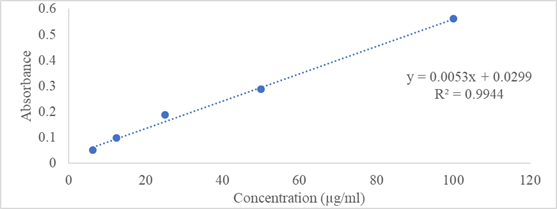
Figure 1: Calibration curve of quercetin
Total phenolic content
The total phenol content in extracts was determined using Folin-Ciocalteu reagent (FCR) based on colorimetric reaction by using Harbourne et al., 2009 method [14]. Gallic Acid was used as standard to produce a calibration curve. The phenol content in extracts was expressed as mg of gallic acid equivalents (GAE) /g of extract (Figure 2).
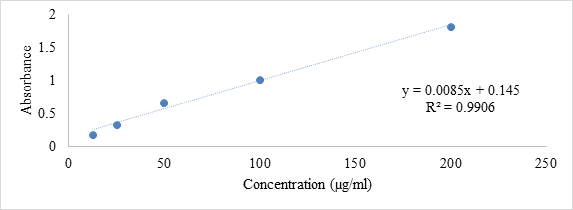
Figure 2: Calibration curve of gallic acid
Total antioxidant capacity
The total antioxidant activity of the extract can be evaluated by the phosphomolybdenum method according to the procedure of Prieto et all. 1999 [15]. The assay is based on the reduction of Mo (VI)-Mo (V) by the extract and subsequent formation of green PO4/Mo (V) complex at acidic PH (Figure 3).
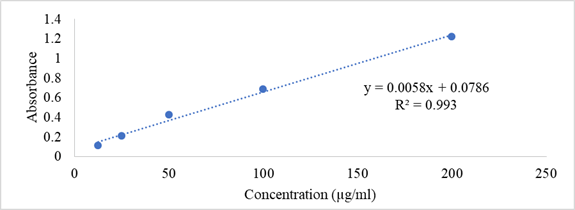
Figure 3: Calibration Curve of Ascorbic Acid
Cytotoxicity (brine shrimp lethality bioassay)
Cytotoxicity of the selected extracts was determined by Brine Shrimp lethality bioassay described by Meyer et all. 1982 [10]. Using this method natural products can be tested for their bioactivity. The method utilizes in vivo lethality in a simple zoological organism (Brine nauplii) as a appropriate monitor for screening and fractionation in the discovery of new bioactive natural products The efficiency or concentration-mortality relationship of the plant products is usually stated as a median lethal concentration (LC50).
The results of antioxidant study of the cure extract and isolated compound are given below (Table 1).
| Extract | Total flavonoids content (mg/g, quercetin equivalents) | Total phenolic content (mg/g, gallic acid equivalents) | Total Antioxidant Capacity (mg/g, Ascorbic Acid Equivalent) |
|---|---|---|---|
| SBEa | 22.26 ± 0.67 | 7.35 ± 1.24 | 8.10± 1.22 |
| E4A | 70.84± 1.33 | 55.29± 1.66 | 37.41± 2.44 |
Table 1: Comparative antioxidants study of fractions SBEa and compound E4A.
The antioxidants study of the ethyl acetate extract and 2',4'-dihydroxy- 3',5'-dimethyl -6'-methoxychalcone exhibited good antioxidant property. The results are also found that 2',4'-dihydroxy-3',5'- dimethyl -6'-methoxychalcone have the higher percentage of total phenols and flavonoids contents. The antioxidant activity of the 2',4'-dihydroxy-3',5'-dimethyl -6'-methoxychalcone showed significant activity compared to ethyl acetate extract.
Characterization of compound E4A
The ethyl acetate extract (SBEa) on repeated column chromatography yielded a new compound E4A from the leaves of Syzygium balsameum, and the compound E4A is known as 2',4'-dihydroxy- 3',5'-dimethyl -6'-methoxychalcone. The identification of the compound was accomplished by comparing their UV, IR, EI MS, 1H and 13C, Cosy, HBMC, HQSC NMR data (Table 2 and 3).
| Proton | Compound E4A | Carbon | Compound E4A |
|---|---|---|---|
| Â a | 8.02 (1H, d, J 15.6Hz) | a | 126.7 |
| Â Ã? | 7.86 (1H, d, J 15.6Hz) | Ã? | 142.9 |
| C=O | 193.4 | ||
| 1' | - | 1' | 108.8 |
| 2' | - | 2' | 162 |
| 3' | - | 3' | 106.5 |
| 4' | - | 4' | 159.2 |
| 5' | - | 5' | 109.1 |
| 6' | - | 6' | 158.9 |
| 1 | - | 1 | 135.4 |
| 2 | 7.68 (1H, m) | 2 | 128.4 |
| 3 | 7.44 (1H, m) | 3 | 128.9 |
| 4 | 7.42 (1H, m) | 4 | 130.5 |
| 5 | 7.42 (1H, m) | 5 | 128.9 |
| 6 | 7.66 (1H, m) | 6 | 128.4 |
| 2' -OH | 13.61 (1H, s) | - | - |
| 4' -OH | 5.44 (1H, s) | - | - |
| 6'-OMe | 3.68 (3H, s) | 6'-OMe | 62.3 |
| 3'-Me | 2.15 (3H, s) | 3'-Me | 7.5 |
| 5'-Me | 2.18 (3H, s) | 5'-Me | 8.2 |
Table 2: 1H and 13C NMR spectral data for Compound E4A, J (Hz) in parentheses.
| 1H | HSQC | HMBC |
|---|---|---|
| 13.6 | - | 108.8, 162.0, 106.5 |
| 8.02 | 126.7 | 193.4, 142.9, 135.4 |
| 7.86 | 142.9 | 193.4, 126.7, 128.4 |
| 7.67 | 128.4 | 130.2, 128.4, 142.9 |
| 7.43 | 128.9, 130.2 | 128.4, 135.4, 128.9 |
| 5.44 | - | 159.2, 109.1, 106.5 |
| 3.68 | 62.3 | 158.9 |
| 2.18 | 8.2 | 108.8, 109.1, 158.9, 159.2 |
| 2.15 | 7.5 | 162.0, 159.2, 106.5 |
Table 3: Long-range correlation of E4A NMR.
Compound E4A was obtained as yellow solid. The EI MS gave a molecular peak at m/z 298, corresponding to the molecular formula C18H18O4. The above data and UV spectrum (λmax) at 339 and 225 nm suggested a chalcone nature for compound E4A [16]. The IR spectrum of the compound E4A showed a broad band at 3419.79 cm-1 suggest the presence of hydrogen bonded hydroxyl (─ OH) groups. Absorption at 1627.92 cm-1 indicates the presence of C = O conjugated with α, β -unsaturated carbons, and (1610, 1541 & 1497 cm-1) for aromatic C=C, which also supported this hypothesis. Another peak at 1167 cm-1 proves the presence CO of groups. The 1HNMR (CDCl3) spectrum indicated the presence of three-proton and two-proton multiples at δ 7.44 and δ 7.67, respectively, which are typical of a flavonoid nucleus with an unsubstituted B ring. An AB spin system (J=15.6 Hz) at δ 7.85 and 8.02 suggested the presence of protons of an α, β -unsaturated ketone moiety. Additionally, the 1H NMR spectrum showed a two-methyl singlet at δ 2.18 and δ 2.15, one methoxy singlet at δ 3.68 and two hydroxyl group singlets at δ 5.44 and 13.61. The 1HNMR spectrum demonstrating signals at the downfield region indicated that the compound may have possessed a 1',2',3',4',5',6'-substituted benzene ring A and a 1-substituted benzene ring B of the chalcone skeleton, respectively. These data indicated that the A-ring of this chalcone may be substituted with two hydroxyl, two methyl and one methoxy groups. The 13CNMR spectrum identified the presence of 18 carbons, three of which were oxyaryl carbons revealed by the signals at δ 158.9, 159.2 and 162.0. These carbon signals indicated the presence of oxygenation in the chalcone skeleton. The existence of a conjugated carbonyl group was indicated by the appearance of the signal at δ 193.4. The 13CNMR data supported the assumption of the substitution pattern of this chalcone compound. The position of the oxygen groups was determined by analysis of the 1H multiplicity bond connectivity (HMBC) data. As indicated in the 1HNMR spectrum, the chalcone skeleton of 2',4'-dihydroxy- 3',5'-dimethyl -6'-methoxychalcone may possess a hydroxyl group chelated to a carbonyl group, indicating that the hydroxyl group was attached to C-2' of the A-ring. Furthermore, the proton signal of methoxy group at δ 3.68 correlated with the carbon signal at δ 158.9 (C-6'), indicating that this compound possessed a methoxy group at C-6'. Thus, from the aforementioned accumulated data, the compound was identified as 2',4'-dihydroxy-3',5'-dimethyl -6'-methoxy-chalcone, which was also confirmed by comparison of its spectral data with the reported data [16](Figure 4and 5).
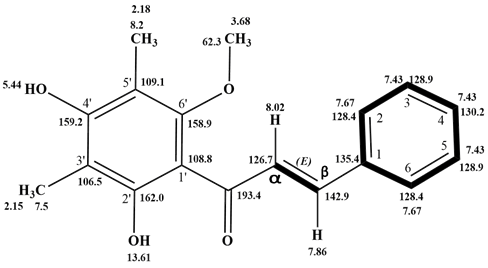
Figure 4: 2',4'-dihydroxy-3',5'-dimethyl -6'-methoxy-chalcone
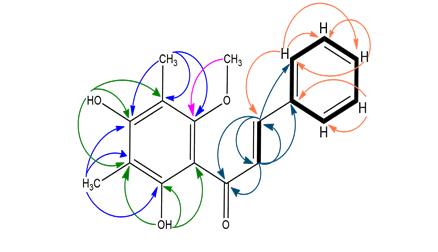
Figure 5: HMBC correlation of 2',4'-dihydroxy-3',5'-dimethyl -6'-methoxy-chalcone
Literature review of the compound 2',4'-dihydroxy-3',5'-dimethyl -6'-methoxy-chalcone found that the compound is highly potential against the different diseases such as antioxidants, anti-diabetics, hepatoprotective and anticancer (26 cell) activity. The compound 2',4'-dihydroxy-3',5'-dimethyl -6'-methoxy-chalcone is found highly potential against different diseases [17-32].
The bioassay guided fractionation led to isolation of the compound 2',4'-dihydroxy-3',5'-dimethyl -6'-methoxy-chalcone is highly potential against several diseases this compound is first compound isolated from the leaves of S. balsameum. The findings of the study suggest that S. balsameum leaves may useful in antioxidants, anti-diabetics, anticancer and hepatoprotective property. More study required to isolate and identified others active components for development of new therapeutic agents.
Received: 11-Jun-2021 Published: 07-Jul-2021, DOI: 10.35248/2329-6836.21.9.409
Copyright:This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.